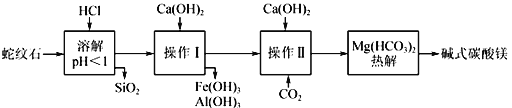
��Ŀ����
����Ŀ���������ڹ�ҵ������Ҫ��;����ͭ������Ǧ�ȵ��յ�����������������Ҫ��������ڻ��P����״̬���ڡ�һ�ִ�����������ȡSe��Te�Ĺ������̼��£�
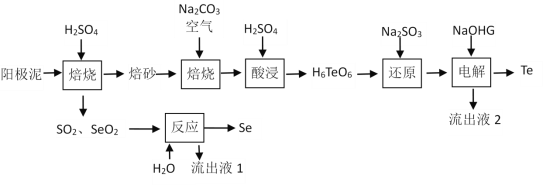
��֪��������(Na2H4TeO6)���ܣ�����(H6TeO6)���ܡ�
�ش��������⣺
��1����������600 K���Ҽ���һ��Ũ��H2SO4��������ʱ������Teת��ΪTeO2�Ļ�ѧ����ʽΪ___________________________________________��
��2�� ����ɰ����̼���Ƴ�ֻ�ϣ������ͨ�����������ϣ�ÿ����1 mol�����ƣ�����Ҫ��������O2����Ϊ_______mol�����ɵ������Ʋ�ˮ��������ȡ���������ԭ����______________________________________��
��3�� ����Ӧ��ʱ�Ļ�ѧ����ʽΪ_________________________________________________��
��4������ԭ���еķ�Ӧ������ΪTeO2�����鷴Ӧ���Ƿ��з�Ӧ��Na2SO3������ʵ���������Ϊ________________________________________________________��
��5����֪�������ʱʹ��ʯī�缫������������������������ʵ���֮��Ϊ______________��
��6������·���п���ѭ�����õ�������_____________________��_____________________��
���𰸡�Te+2H2SO4![]() TeO2+2SO2��+2H2O0.5Na2H4TeO6 ��ˮ�����ܣ�������ɵ�H6TeO6����SeO2+2H2O+2SO2==2H2SO4+Seȡ��������ԭ�������Һ���Թܣ�����ϡH2SO4�������ɵ�����ͨ�����ʯ��ˮ������Һ����ǣ���Na2SO3��������֮������1��1H2SO4NaOH
TeO2+2SO2��+2H2O0.5Na2H4TeO6 ��ˮ�����ܣ�������ɵ�H6TeO6����SeO2+2H2O+2SO2==2H2SO4+Seȡ��������ԭ�������Һ���Թܣ�����ϡH2SO4�������ɵ�����ͨ�����ʯ��ˮ������Һ����ǣ���Na2SO3��������֮������1��1H2SO4NaOH
��������
(1)��������600K���Ҽ���һ��Ũ��H2SO4����ʱ������Teת��ΪTeO2,ͬʱ���ɶ��������ˮ����Ӧ�Ļ�ѧ����ʽΪTe+2H2SO4![]() TeO2+2SO2��+2H2O��(2)����ɰ����̼���Ƴ�ֻ�ϣ������ͨ��������գ�����������Ӧ2Na2CO3+O2+ 2TeO2=2Na2H4TeO6+2CO2��ÿ����1mol����������(Na2H4TeO6)������Ҫ��������O2����Ϊ0.5mol��Na2H4TeO6��ˮ�����ܣ�������ɵ�H6TeO6���ܣ������ɵ����������Ʋ��á�ˮ����������ȡ��������� (3)�����С���Ӧ����ͨ��SeO2��SO2������H2O��Ӧ��õ�Se����SO2�������������ᣬ������Ӧ�Ļ�ѧ����ʽΪSeO2+2H2O+2SO2=2H2SO4+Se��(4)���顰��ԭ�������Һ��Na2SO3�Ƿ������ʵ���������Ϊȡ��������ԭ�������Һ���Թܣ�����ϡH2SO4�����ɵ�����������ʯ��ˮ������Һ����ǣ���Na2SO3��������֮��������(5) �������ʱʹ��ʯī�缫�������������������������Te���ʵ���֮��Ϊ1��1����6�����������м�Ϊ��������Ϊ��Ӧԭ�ϵ����ʿ���Ϊѭ�����õ����ʣ�����·���п���ѭ�����õ�������H2SO4 ��NaOH��
TeO2+2SO2��+2H2O��(2)����ɰ����̼���Ƴ�ֻ�ϣ������ͨ��������գ�����������Ӧ2Na2CO3+O2+ 2TeO2=2Na2H4TeO6+2CO2��ÿ����1mol����������(Na2H4TeO6)������Ҫ��������O2����Ϊ0.5mol��Na2H4TeO6��ˮ�����ܣ�������ɵ�H6TeO6���ܣ������ɵ����������Ʋ��á�ˮ����������ȡ��������� (3)�����С���Ӧ����ͨ��SeO2��SO2������H2O��Ӧ��õ�Se����SO2�������������ᣬ������Ӧ�Ļ�ѧ����ʽΪSeO2+2H2O+2SO2=2H2SO4+Se��(4)���顰��ԭ�������Һ��Na2SO3�Ƿ������ʵ���������Ϊȡ��������ԭ�������Һ���Թܣ�����ϡH2SO4�����ɵ�����������ʯ��ˮ������Һ����ǣ���Na2SO3��������֮��������(5) �������ʱʹ��ʯī�缫�������������������������Te���ʵ���֮��Ϊ1��1����6�����������м�Ϊ��������Ϊ��Ӧԭ�ϵ����ʿ���Ϊѭ�����õ����ʣ�����·���п���ѭ�����õ�������H2SO4 ��NaOH��

����Ŀ����֪��������ĵ���ƽ�ⳣ�����±���
���� | ���� | ������ | ̼�� | ������ |
����ƽ�ⳣ����25���� | Ka=1.75��10��5 | Ka=2.98��10��8 | Ka1=4.30��10��7 Ka2=5.61��10��11 | Ka1=1.54��10��2 Ka2=1.02��10��7 |
�������ӷ���ʽ��ȷ����
A������CO2ͨ��NaClO��Һ�У�CO2��H2O��2ClO����CO![]() ��2HClO
��2HClO
B��������SO2ͨ��Ca(ClO)2��Һ�У�SO2��H2O��Ca2����2ClO����CaSO3����2HClO
C��������SO2ͨ��Na2CO3��Һ�У�SO2��H2O��2 CO![]() ��SO
��SO![]() ��2HCO3��
��2HCO3��
D����ͬŨ��NaHCO3��Һ��NaHSO3��Һ�������ϣ�H+��HCO3����CO2����H2O