ЬтФПФкШн
ЁОЬтФПЁПТШЫсЪЧвЛжжЧПЫсЃЌХЈЖШГЌЙ§40%ЪБЛсЗЂЩњЗжНтЃЌЗДгІПЩБэЪОЮЊЃКaHClO3ЈTbO2Ёќ+cCl2Ёќ+dHClO4+eH2OЃЌгУЪЊШѓЕФЕэЗлЕтЛЏМиЪджНМьбщЦјЬхВњЮяЪБЃЌЪджНЯШБфРЖКѓЭЪЩЋЁЃЯТСаЫЕЗЈе§ШЗЕФЪЧ( )
A.гЩЗДгІПЩШЗЖЈЃКбѕЛЏадЃКHClO4ЃОHClO3
B.БфРЖЕФЕэЗлЕтЛЏМиЪджНЭЪЩЋЪЧвђЮЊПЩФмЗЂЩњСЫЃК4Cl2+I2+6H2OЈT12H++8Cl-+2IO3-
C.ШєТШЫсЗжНтЫљЕУЛьКЯЦјЬхЃЌ1 molЛьКЯЦјЬхжЪСПЮЊ47.6 gЃЌдђЗДгІЗНГЬЪНПЩБэЪОЮЊ26HClO3 ЈT15O2Ёќ+8Cl2Ёќ+10HClO4+8H2O
D.ШєЛЏбЇМЦСПЪ§a=8ЃЌb=3ЃЌдђИУЗДгІзЊвЦЕчзгЪ§ЮЊ20e-
ЁОД№АИЁПD
ЁОНтЮіЁП
A. aHClO3ЈTbO2Ёќ+cCl2Ёќ+dHClO4+eH2OЗДгІжаЃЌHClO3ЪЧбѕЛЏМСЃЌHClO4ЁЂO2ЪЧбѕЛЏВњЮяЃЌЫљвдбѕЛЏадЃКHClO3ЃОHClO4ЃЌЙЪAДэЮѓЃЛ
B. БфРЖЕФЕэЗлЕтЛЏМиЪджНЭЪЩЋЪЧвђЮЊI2БЛCl2МЬајбѕЛЏЩњГЩIO3-ЃК5C12+I2+6H2O=12H++10Cl-+2IO3-ЃЌЙЪBДэЮѓЃЛ
C. гЩЩњГЩЕФCl2КЭO2ЕФЛьКЯЦјЬхЦНОљЗжзгСПЮЊ47.6g/molЃЌдђ![]() ЃЌПЩЕУnЃЈCl2ЃЉЃКnЃЈO2ЃЉ=2ЃК3ЃЌгЩЕчзгЪиКуЕУЛЏбЇЗДгІЗНГЬЪНЮЊ8 HClO3=3O2Ёќ+2 Cl2Ёќ+4 HClO4+2H2OЃЌЙЪCДэЮѓЃЛ
ЃЌПЩЕУnЃЈCl2ЃЉЃКnЃЈO2ЃЉ=2ЃК3ЃЌгЩЕчзгЪиКуЕУЛЏбЇЗДгІЗНГЬЪНЮЊ8 HClO3=3O2Ёќ+2 Cl2Ёќ+4 HClO4+2H2OЃЌЙЪCДэЮѓЃЛ
D. ШєЛЏбЇМЦСПЪ§a=8ЃЌb=3ЃЌгЩCПЩжЊЃЌЛЏбЇЗДгІЗНГЬЪНЮЊ8 HClO3=3O2Ёќ+2 Cl2Ёќ+4 HClO4+2H2OЃЌЕчзгзЊвЦЪ§ЮЊ20e-ЃЌЙЪDе§ШЗЃЛД№АИбЁDЁЃ

 ЦкФЉМЏНсКХЯЕСаД№АИ
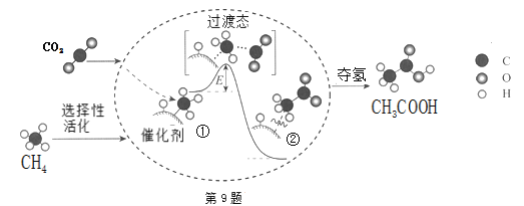
ЦкФЉМЏНсКХЯЕСаД№АИЁОЬтФПЁПЪЏгЭзЪдДНєеХдјЪЧжЦдМжаЙњЗЂеЙНЮГЕЪТвЕЃЌгШЦфЪЧжЦдМНЮГЕНјШыМвЭЅЕФживЊвђЫиЁЃОнБЈЕРЃЌжаЙњаћВМНЋЭЦЙуЁАГЕгУввДМЦћгЭЁБЁЃ
ЃЈ1ЃЉввДМШМЩеЪБШчЙћбѕЦјВЛзуЃЌПЩФмЛЙгаCOЩњГЩЃЎгУЯТЭМзАжУе§ШЗбщжЄввДМШМЩеВњЮягаCOЁЂ![]() ЁЂ
ЁЂ![]() ЃЌгІНЋввДМШМЩеВњЮявРДЮЭЈЙ§ЃЌАДЦјСїДгзѓжСгвЫГађЬюзАжУБрКХ______ЁЃ
ЃЌгІНЋввДМШМЩеВњЮявРДЮЭЈЙ§ЃЌАДЦјСїДгзѓжСгвЫГађЬюзАжУБрКХ______ЁЃ
БрКХ | Ђй | Ђк | Ђл | Ђм |
зАжУ |
|
|
|
|
ЃЈ2ЃЉЪЕбщЪБПЩЙлВьЕНзАжУЂкжаAЦПЕФЪЏЛвЫЎБфЛызЧЁЃAЦПШмвКЕФзїгУЪЧ______ЃЛBЦПШмвКЕФзїгУЪЧ______ЁЃ
ЃЈ3ЃЉзАжУЂйжаЫљЪЂЕФЪЧ______ШмвКЃЌжЄУїШМЩеВњЮяжаКЌгаCOЕФЪЕбщЯжЯѓЪЧЃК______ЁЃ
ЃЈ4ЃЉзАжУЂмжаЫљЪЂЕФЙЬЬхвЉЦЗЪЧ______ЃЌЫќПЩвдбщжЄЕФВњЮяЪЧ______ЁЃ
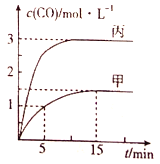
ЁОЬтФПЁПвЛЖЈЬѕМўЯТДцдкЗДгІЃКC(s)+H2O(g)![]() CO(g)+H2(g) ЁїH>0ЃЌЯђМзЁЂввЁЂБћШ§ИіКуШнШнЦїжаМгШывЛЖЈСПЕФГѕЪМЮяжЪЃЌИїШнЦїжаЮТЖШЁЂЗДгІЮяЕФЦ№ЪМСПШчБэЃЌЗДгІЙ§ГЬжаCOЕФЮяжЪЕФСПХЈЖШЫцЪБМфБфЛЏШчЭМЫљЪОЃК
CO(g)+H2(g) ЁїH>0ЃЌЯђМзЁЂввЁЂБћШ§ИіКуШнШнЦїжаМгШывЛЖЈСПЕФГѕЪМЮяжЪЃЌИїШнЦїжаЮТЖШЁЂЗДгІЮяЕФЦ№ЪМСПШчБэЃЌЗДгІЙ§ГЬжаCOЕФЮяжЪЕФСПХЈЖШЫцЪБМфБфЛЏШчЭМЫљЪОЃК
ШнЦї | Мз | вв | Бћ |
|
ШнЛ§/L | 1 | 1 | V | |
ЮТЖШ/Ёц | T1 | T2 | T1 | |
Ц№ЪМСП | 2 molC(s)ЁЂ2 mol H2O(g) | 2 mol CO(g)ЁЂ2 mol H2(g) | 6 molC(s)ЁЂ 4mol H2O(g) |
ЯТСаЫЕЗЈе§ШЗЕФЪЧ( )
A.МзШнЦїжаЃЌ0ЁЋ5minФкЕФЦНОљЗДгІЫйТЪv(CO)=0.1 mol/(LЁЄmin)
B.ввШнЦїжаЃЌШєЦНКтЪБn(C)=0.56 molЃЌдђT2>T1
C.ЮТЖШЮЊT1ЪБЃЌЗДгІЕФЦНКтГЃЪ§ЮЊK=9
D.БћШнЦїЕФШнЛ§V=0.8 L