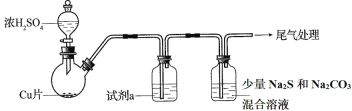
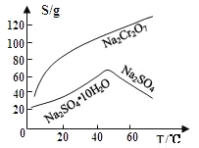
��Ŀ����
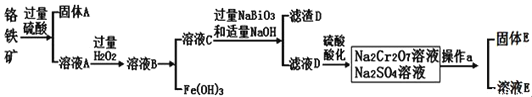
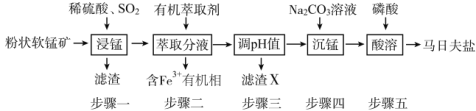
����Ŀ�����շ���[Mn(H2PO4)2��2H2O]��Ҫ���������������̿�(��Ҫ�ɷ�ΪMnO2��������FeO��Al2O3��SiO2)Ϊԭ���Ʊ����շ��ε���Ҫ����������ͼ��
��1�����������������ɣ����շ���[Mn(H2PO4)2��2H2O�Ļ�ѧ����Ϊ___��
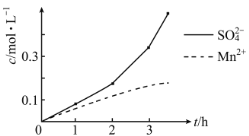
��2������һ�У�MnO2��SO2��___��ԭ����SO2�����л��п�������÷�ӦҺ��Mn2+��SO42����Ũ���淴Ӧʱ��t�仯��ͼ��������Ա��ΪMn2+��O2��H2SO3��Ӧ������ã���������__��
��3������X��Ҫ�ɷ�Ϊ___������������pHʱ�������˼����������__��
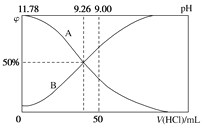
A������ϡ���� B������Na2CO3��Һ
C������������Һ D������CaCO3
��4���ڳ��̹����У�Ӧ��Na2CO3��Һ��������������Һ�У�����ߵ��Լ����˳�����Mn(OH)2���ɣ���ԭ����Na2CO3��Һ���н�ǿ__�ԣ�������������շ��ξ���Ļ�ѧ��Ӧ����ʽΪ__��
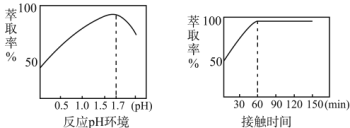
��5��Fe3+����ȡ������Һ��pH�ͽӴ�ʱ��֮��Ĺ�ϵ��ͼ���ݴ˷�������ȡ���������Ϊ____��
��6�����շ���������������ԭ�����������ǿ�������Լ��ڷ���������������˾��б������õ�FeHPO4�����շ��������Ե���Ҫԭ����__(����ػ�ѧ����ش�)��
���𰸡���ˮ����������� FeO Mn2+���ӵ�һ��Ũ�Ⱥ������Ũ��Ѹ������ �������� B �� MnCO3 + 2 H3PO4 + H2O = Mn(H2PO4)2��2H2O + CO2�� pH =1.7����ȡ60min H2PO4�� ![]() H+ + HPO42�������������������ˮ�⣬�����Һ������
H+ + HPO42�������������������ˮ�⣬�����Һ������
��������
�Ű��������������ɣ����շ���Mn(H2PO4)2��2H2O�Ļ�ѧ����Ϊ��ˮ����������̡�
�Ʋ���һ�У����̿��ж�����������������������������������ԭ��Ӧ�����MnO2��SO2��FeO��ԭ����SO2�����л��п�������÷�ӦҺ��Mn2+��SO42����Ũ���淴Ӧʱ��t�仯��ͼ������ͼ������ó��ڿ�ʼ�Σ������Ӻ��������ͬ����������������������������������ٶȿ죬���Mn2+��O2��H2SO3��Ӧ������á�
�Dz���һ����Ϊ����SiO2����������������ӣ�������������Ϊ��������������������pHʱ��Aѡ�����ϡ���ᣬ���ܵ���pH����Bѡ�����Na2CO3��Һ�����������ӣ�pH����Cѡ�����������Һ�����ܵ���pH����Dѡ�����CaCO3�����������ӣ�pH������������������ʣ����B��ȷ��
���ڳ��̹����У�Ӧ��Na2CO3��Һ��������������Һ�У�����ߵ��Լ����˳�����Mn(OH)2���ɣ���ԭ����Na2CO3��Һˮ�⣬��Һ���н�ǿ���ԣ�������������շ��ξ���Ļ�ѧ��Ӧ����ʽΪMnCO3 + 2 H3PO4 + H2O = Mn(H2PO4)2��2H2O + CO2����
��Fe3+����ȡ������Һ��pH�ͽӴ�ʱ��֮��Ĺ�ϵ��ͼ������ͼ����Ϣ�ó���ȡ���������ΪpH =1.7����ȡ60min��
�����շ���������������ԭ�����������ǿ�������Լ��ڷ���������������˾��б������õ�FeHPO4�����շ��������Ե���Ҫԭ����H2PO4�� ![]() H+ + HPO42�������������������ˮ�⣬�����Һ�����ԡ�
H+ + HPO42�������������������ˮ�⣬�����Һ�����ԡ�
�Ű��������������ɣ����շ���Mn(H2PO4)2��2H2O�Ļ�ѧ����Ϊ��ˮ����������̣��ʴ�Ϊ����ˮ����������̡�
�Ʋ���һ�У����̿��ж�����������������������������������ԭ��Ӧ�����MnO2��SO2��FeO��ԭ����SO2�����л��п�������÷�ӦҺ��Mn2+��SO42����Ũ���淴Ӧʱ��t�仯��ͼ������ͼ������ó��ڿ�ʼ�Σ������Ӻ��������ͬ����������������������������������ٶȿ죬���Mn2+��O2��H2SO3��Ӧ������ã��ʴ�Ϊ��FeO��Mn2+���ӵ�һ��Ũ�Ⱥ������Ũ��Ѹ������
�Dz���һ����Ϊ����SiO2����������������ӣ�������������Ϊ��������������������pHʱ��Aѡ�����ϡ���ᣬ���ܵ���pH����Bѡ�����Na2CO3��Һ�����������ӣ�pH����Cѡ�����������Һ�����ܵ���pH����Dѡ�����CaCO3�����������ӣ�pH������������������ʣ��ʴ�Ϊ������������B��
���ڳ��̹����У�Ӧ��Na2CO3��Һ��������������Һ�У�����ߵ��Լ����˳�����Mn(OH)2���ɣ���ԭ����Na2CO3��Һˮ�⣬��Һ���н�ǿ���ԣ�������������շ��ξ���Ļ�ѧ��Ӧ����ʽΪMnCO3 + 2 H3PO4 + H2O = Mn(H2PO4)2��2H2O + CO2�����ʴ�Ϊ���MnCO3 + 2 H3PO4 + H2O = Mn(H2PO4)2��2H2O + CO2����
��Fe3+����ȡ������Һ��pH�ͽӴ�ʱ��֮��Ĺ�ϵ��ͼ������ͼ����Ϣ�ó���ȡ���������ΪpH =1.7����ȡ60min���ʴ�Ϊ��pH =1.7����ȡ60min��
�����շ���������������ԭ�����������ǿ�������Լ��ڷ���������������˾��б������õ�FeHPO4�����շ��������Ե���Ҫԭ����H2PO4�� ![]() H+ + HPO42�������������������ˮ�⣬�����Һ�����ԣ��ʴ�Ϊ��H2PO4��
H+ + HPO42�������������������ˮ�⣬�����Һ�����ԣ��ʴ�Ϊ��H2PO4�� ![]() H+ + HPO42�������������������ˮ�⣬�����Һ�����ԡ�
H+ + HPO42�������������������ˮ�⣬�����Һ�����ԡ�

����Ŀ��Ϊ�ﵽ����ʵ��Ŀ�ģ���Ӧ��ʵ�鷽���Լ���ؽ��;���ȷ���ǣ� ��
ѡ�� | ʵ��Ŀ�� | ʵ�鷽�� | ��ؽ��� |
A | ������ˮ��pH |
| pH��ֽ������ |
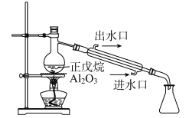
B | ̽��������(C5H12) ���ѽ� |
| C5H12�ѽ�Ϊ���ӽ�С��������ϩ�� |
C | ʵ���¶ȶ�ƽ���ƶ���Ӱ�� |
| 2NO2(g) |
D | ��AlCl3��Һ�Ʊ�AlCl3���� |
| AlCl3�е�����ܼ�ˮ |
A.AB.BC.CD.D