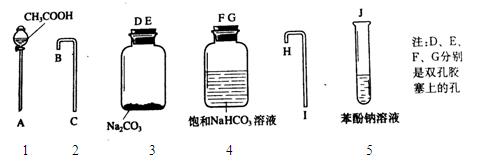
��Ŀ����
��14�֣�ij������ȤС���ͬѧ��Na2SiO3��Һ�еμ����ᣬԤ�ƻ�����H2SiO3���壬�ü�����������֡�������������������벻�����ǣ��Թ����ڳ��ֹ��ὺ���ͬʱҲ�����˴������ݣ�Ϊʲô��������������أ�������ͬѧ�����˴��Ʋ⡣
��ͬѧ�Ʋ��������˹��ὺ�壬���HCl����Һ�е��ܽ�ȱ�С�����մ���Һ���ݳ���
��ͬѧ��ѯ��ʵ��Ա��֪��ƿNa2SiO3��Һ�Ѿ����úܳ�һ��ʱ�䣬���Ʋ���Һ�����Ѿ����ʣ��ʵ�����������ij��֡�
��1�������һ�����ʵ����֤��ͬѧ���Ʋ��Ƿ���ȷ�� ��
��2����ͬѧȡ��ƿ���õĹ�������Һ���ü�������䣬�����й������֣�˵������ƿ��������Һ���Ѿ��� �������ɡ��Ʋ��������������� ��Ȼ����ͬѧ����������ʵ�������֤��
Iȡ������ƿ��Һ�������еμ� ����������������塣��д�����������ij��÷����� ��
IIȡ�����ƹ��壬��ɱ�����Һ���������ã��������ᣬ�۲�����
ͨ������ʵ�飬֤������ͬѧ�۵㣬��д������Ӧ�Ļ�ѧ��Ӧ����ʽ��
�� ��
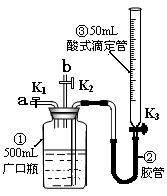
��С��ͬѧ�����ְ��̲������IJ��������������Ʊ����ὺ�壬��ʱ��Ҫ�ܳ�ʱ�����ʹ�ܽ��������ۣ����������γ�������Ч���ܲ����롣��ˣ�����ͬѧ�ֶ�ʵ�������Ͳ�������̽�������ʵ���������±���
���鲿��ͬѧͨ��ʵ��ó����ۣ�ʹ�ñ���Na2SiO3��Һ��ϡ���ᣬ��Na2SiO3��Һ�μӵ�ϡ������Ч���Ϻá�����������һ����ͬѧ��Ϊ����������������̽��ʵ������������������ܱ�֤���۵�ȷ�ԣ�Ҫʹʵ�����˵��������Ҫ����ʵ�顣
��ͬѧ�Ʋ��������˹��ὺ�壬���HCl����Һ�е��ܽ�ȱ�С�����մ���Һ���ݳ���
��ͬѧ��ѯ��ʵ��Ա��֪��ƿNa2SiO3��Һ�Ѿ����úܳ�һ��ʱ�䣬���Ʋ���Һ�����Ѿ����ʣ��ʵ�����������ij��֡�
��1�������һ�����ʵ����֤��ͬѧ���Ʋ��Ƿ���ȷ�� ��
��2����ͬѧȡ��ƿ���õĹ�������Һ���ü�������䣬�����й������֣�˵������ƿ��������Һ���Ѿ��� �������ɡ��Ʋ��������������� ��Ȼ����ͬѧ����������ʵ�������֤��
Iȡ������ƿ��Һ�������еμ� ����������������塣��д�����������ij��÷����� ��
IIȡ�����ƹ��壬��ɱ�����Һ���������ã��������ᣬ�۲�����
ͨ������ʵ�飬֤������ͬѧ�۵㣬��д������Ӧ�Ļ�ѧ��Ӧ����ʽ��
�� ��
��С��ͬѧ�����ְ��̲������IJ��������������Ʊ����ὺ�壬��ʱ��Ҫ�ܳ�ʱ�����ʹ�ܽ��������ۣ����������γ�������Ч���ܲ����롣��ˣ�����ͬѧ�ֶ�ʵ�������Ͳ�������̽�������ʵ���������±���
| | ��������ҺŨ�� | ����Ũ�� | �Լ�����˳�� | ��������ʱ�䣨s�� |
| ʵ��1 | ������Һ | 1��2�������ˮ����ȣ���ͬ�� | ��Na2SiO3��Һ�μӵ�ϡ������ | 21 |
| ʵ��2 | ϡ��Һ��1���������Һ��3���ˮ��ϣ� | 1��2 | ��ϡ����μӵ�Na2SiO3��Һ�� | 38 |
| ʵ��3 | ������Һ | 1��4 | ��Na2SiO3��Һ�μӵ�ϡ������ | 10 |
| ʵ��4 | ϡ��Һ��1���������Һ��3���ˮ��ϣ� | 1��4 | ��ϡ����μӵ�Na2SiO3��Һ�� | 50 |
��14�֣�


��

��ϰ��ϵ�д�
�����Ŀ

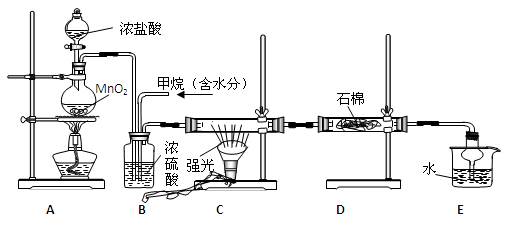
 ����Ӧ����Ʋ��������ͼ��ʾ
����Ӧ����Ʋ��������ͼ��ʾ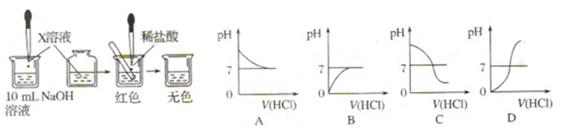
 �Թ��У��ý�ͷ�ι���������ϡ���ᣬ��������Һ��ͬʱ�ⶨ��Һ��pH��ֱ�����������
�Թ��У��ý�ͷ�ι���������ϡ���ᣬ��������Һ��ͬʱ�ⶨ��Һ��pH��ֱ����������� )/mL
)/mL ������������������֮��ı仯��ϵͼ�����������ϻ����ܱ�ʾ����֮��ǡ����ȫ��Ӧ�ĵ㣬������ĸP��ʾ��
������������������֮��ı仯��ϵͼ�����������ϻ����ܱ�ʾ����֮��ǡ����ȫ��Ӧ�ĵ㣬������ĸP��ʾ��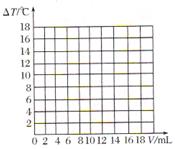
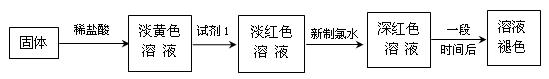
 ������һ����̽��С�������ѧ֪ʶ���������Ҵ���ˮ�����ӡ�̼�ᣨ HO��C��OH��������������ǻ�����ԭ�ӵĻ����ԡ� O
������һ����̽��С�������ѧ֪ʶ���������Ҵ���ˮ�����ӡ�̼�ᣨ HO��C��OH��������������ǻ�����ԭ�ӵĻ����ԡ� O