��Ŀ����
��12�֣���ѧ�о���ѧϰС����ͨ��ʵ��̽�������ƵĻ�ԭ�����ۺ������ᷴӦ����FeCl2����FeCl3�����������̽�����ش��й����⣺
��1��һλͬѧ����Ӧ�����Һ�еμ�NaOH��Һ�ķ�������֤��Һ�к���Fe2+���ٿɹ۲쵽��ʵ��������___________���ڷ�Ӧ�����з�����Ӧ�Ļ�ѧ����ʽ�� ��
��2����һλͬѧ��Ӧ�����Һ���ȵμ�KSCN��Һ���������еμ�������ˮ����Һ���ֺ�ɫ�������μӹ���������ˮʱ��ȴ���ֺ�ɫ��ȥ��Ϊ��Ū����Һ��ɫ��ȥ��ԭ��ͬѧ�Dz鵽�������ϣ�
������һ�ֻ������Ϊ�����Σ�����FeO42-����
��SCN���ĵ���ʽΪ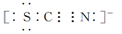
III����ˮ���к�ǿ�������ԡ�
����ͬѧ��������ּ��裺
�ٵ�һ�ּ����ǣ�Cl2�ɽ�Fe3+����ΪFeO42-����д�������ӷ�Ӧ����ʽ__________��
�ڵڶ��ּ����ǣ�____________������ü��������������___________________
��1��һλͬѧ����Ӧ�����Һ�еμ�NaOH��Һ�ķ�������֤��Һ�к���Fe2+���ٿɹ۲쵽��ʵ��������___________���ڷ�Ӧ�����з�����Ӧ�Ļ�ѧ����ʽ�� ��
��2����һλͬѧ��Ӧ�����Һ���ȵμ�KSCN��Һ���������еμ�������ˮ����Һ���ֺ�ɫ�������μӹ���������ˮʱ��ȴ���ֺ�ɫ��ȥ��Ϊ��Ū����Һ��ɫ��ȥ��ԭ��ͬѧ�Dz鵽�������ϣ�
������һ�ֻ������Ϊ�����Σ�����FeO42-����
��SCN���ĵ���ʽΪ
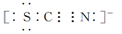
III����ˮ���к�ǿ�������ԡ�
����ͬѧ��������ּ��裺
�ٵ�һ�ּ����ǣ�Cl2�ɽ�Fe3+����ΪFeO42-����д�������ӷ�Ӧ����ʽ__________��
�ڵڶ��ּ����ǣ�____________������ü��������������___________________
��1���ٲ�����ɫ������Ѹ�ٱ�Ϊ����ɫ�����ձ�ɺ��ɫ��
��FeCl2��2NaOH===Fe��OH��2����2NaCl
4Fe��OH��2��O2��2H2O===4Fe��OH��3��
��2����2Fe3����3Cl2��8H2O===2FeO��6Cl����16H����
��SCN����Cl2�������ӵ���ʽ������SCN����SΪ�����ۣ�NΪ�����ۣ���Ϊ��ͼ��б������Ŀ���
��FeCl2��2NaOH===Fe��OH��2����2NaCl
4Fe��OH��2��O2��2H2O===4Fe��OH��3��
��2����2Fe3����3Cl2��8H2O===2FeO��6Cl����16H����
��SCN����Cl2�������ӵ���ʽ������SCN����SΪ�����ۣ�NΪ�����ۣ���Ϊ��ͼ��б������Ŀ���
��������Ԫ�ؼ��仯�����֪ʶ��Ϊ���������鷽��ʽ��д���������Լ�������⣬�������⣬��������������
��Fe2����2OH�D=Fe(OH)2����4Fe��OH��2��O2��2H2O===4Fe��OH��3���ٲ�����ɫ������Ѹ�ٱ�Ϊ����ɫ�����ձ�ɺ��ɫ,�ڷ���ʽ��FeCl2��2NaOH===Fe��OH��2����2NaCl��
4Fe��OH��2��O2��2H2O===4Fe��OH��3��Cl2�ɽ�Fe3+������2����2Fe3����3Cl2��8H2O===2FeO��6Cl����16H���� ��SCN����Cl2�������ӵ���ʽ������SCN����SΪ�����ۣ�NΪ�����ۣ���Ϊ��ͼ��б������Ŀ��ܡ�

��ϰ��ϵ�д�
 С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д� һ������ϵ�д�
һ������ϵ�д� �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д�
�����Ŀ
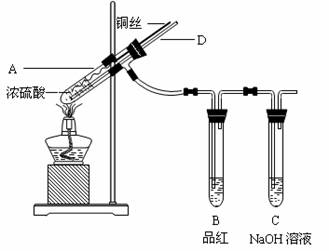
 ��
��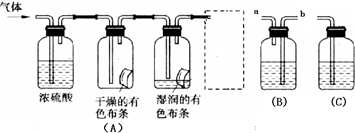
 l2����Ư���ԡ�
l2����Ư���ԡ�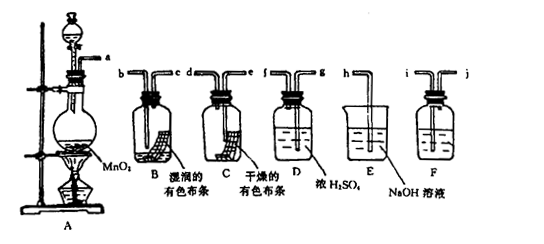
 ��
�� ��ɫ����������ɵó��Ľ�����
��ɫ����������ɵó��Ľ����� 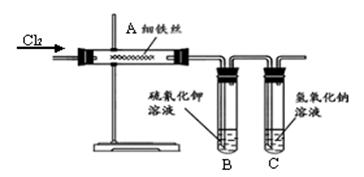
 �Թ�B�е�������___________________��
�Թ�B�е�������___________________��
 FeSO4��Һ
FeSO4��Һ FeSO4��7H2O����
FeSO4��7H2O����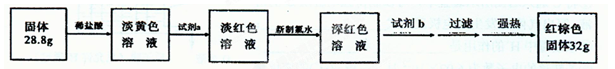
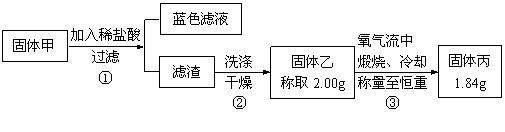
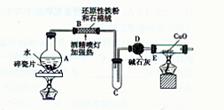
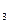 ������������ȷ��̼��Ƶ�����������
������������ȷ��̼��Ƶ�����������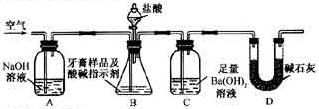
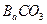 ������ֻҪ�ⶨװ��C������
������ֻҪ�ⶨװ��C������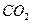 ǰ��������һ������ȷ��̼��Ƶ�����������ʵ��֤�����˷����ⶨ�Ľ������ƫ�ߣ�ԭ����______.
ǰ��������һ������ȷ��̼��Ƶ�����������ʵ��֤�����˷����ⶨ�Ľ������ƫ�ߣ�ԭ����______.