��Ŀ����
����Ŀ����ˮú���ǹ�ҵ�ϳɰ���ԭ����������Ҫ�ɷ���H2��CO��CO2��N2��H2O��g������ˮú���������в���ת��Ϊ�ϳɰ���ԭ�ϡ�
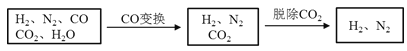
���������գ�
��1����ˮú�������������⡣����ˮú����Ʒͨ��____��Һ�У���д�Լ����ƣ�������_______������֤����������ڡ�
��2����ˮú����ͭ����ʵ��CO�任��CO+H2O![]() CO2+H2
CO2+H2
����ˮú����V(H2):V(CO):V(N2)=38��28��22����CO�任��������У�V(H2):V(N2)=____________��
��3����Һ���շ����ѳ�������̼�ķ���֮һ����֪��
Na2CO3 | K2CO3 | |
20����Һ���Ũ�ȣ�mol/L�� | 2.0 | 8.0 |
��ļ۸�Ԫ/kg�� | 1.25 | 9.80 |
��ѡ��Na2CO3��Һ������Һ�����ŵ���__________��ȱ����____________�����ѡ��K2CO3��Һ������Һ����ʲô�������Խ��ͳɱ���
___________________________________________
д�����ַ����漰�Ļ�ѧ��Ӧ����ʽ��_______________________
��4�������Dzⶨ��ˮú����H2�Լ�CO�����������ʵ�鷽����
ȡһ���������״�����İ�ˮú������������ʵ�鲽��ⶨ����H2�Լ�CO�����������
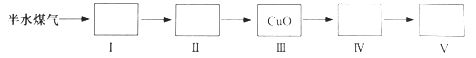
��ѡ�ú��ʵ����Լ��ֱ������������������������С�
����ʵ�鷽���У�������������Ŀ���ǣ�_________________��
����ʵ�鷽���У�����________��ѡ����������������������ȷ����ˮú����H2�����������
���𰸡���1������Ǧ��������ͭ������ɫ����
��2��3:1
��3������������CO2������
��Һѭ��ʹ�ã�2KHCO3��K2CO3+CO2��+H2O
��4����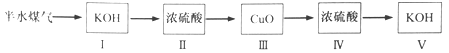
����ȥ��ˮú���е�CO2������H2S����H2O ��IV
�������������������1�����������ؽ������ɳ��������Խ���ˮú����Ʒͨ������Ǧ��������ͭ����Һ�У����ֺ�ɫ��������֤����������ڡ�
��2������ˮú����V(H2):V(CO):V(N2)=38��28��22����CO�任��H2Oת��Ϊ����������ݷ���ʽ��֪���õ������У�V(H2):V(N2)=��38+28����22��3:1��
��3�����ݱ������ݿ�֪��ѡ��Na2CO3��Һ������Һ�����ŵ��Ǽ�������ȱ��������CO2������������ɵ�̼����������ֽ����̼��أ�����ʹ��Һѭ��ʹ�ÿ��Խ��ͳɱ�����Ӧ�Ļ�ѧ����ʽΪ2KHCO3��K2CO3+CO2��+H2O��
��4�������ڰ�ˮú���к��ж�����̼�������������ü�Һ��ȥ������̼���������ͨ������ͭ��Ӧ������Ũ�������ղ�����ˮ���������ü�Һ���ղ����Ķ�����̼���������������������������Ϊ
 ��
��
�ڢ���KOH����CO2��H2S������Ũ��������ˮ�������������塣
��������ԭ����ͭ����ˮ������Ũ��������ˮ���������Ը�ʵ�鷽���У�����������ȷ����ˮú����H2�����������