��Ŀ����
����Ŀ��A��B��C��D��E��F��G����ǰ������Ԫ����ԭ��������������A��������ۺ�����۵ľ���ֵ��ȣ�B�Ļ�̬ԭ����3����ͬ���ܼ��Ҹ��ܼ��е�������ȣ�D�Ļ�̬ԭ����B�Ļ�̬ԭ�ӵ�δ�ɶԵ�����Ŀ��ͬ��E�Ļ�̬ԭ��s�ܼ��ĵ���������p�ܼ��ĵ�������ȣ�F�Ļ�̬ԭ�ӵ�3d�����������4s��������5����G��3d�����3��δ�ɶԵ��ӣ���ش��������⣺
��1��F�Ļ�̬ԭ�ӵ����Ų�ʽΪ ��G�����ڱ���λ�� ��
��2��B��C��D��ԭ�ӵĵ�һ��������С�����˳��Ϊ ����Ԫ�ط��Żش𣩣�A��C��D�γɵ����ӻ������еĻ�ѧ������ ��
��3�����й���B2A2��Bԭ�����������8�����ȶ��ṹ�����Ӻ�A2D2���ӵ�˵����ȷ���� ��
a�������ж��������������� b��B2A2���ӵķе����Ե���A2D2����
c�����Ǻ����Լ��ͷǼ��Լ��ķǼ��Է��� d����Ϊ�ȵ����壬���ӵĿռ乹�Ͷ�Ϊֱ����
e������ԭ�Ӷ���sp�ӻ�
��4���õ���ʽ��ʾE���Ȼ�����γɹ��� ��
��5��F���������Ϊ+6�ۣ�����ԭ�����ֻ���γ�2�����ۼ������Ʋ�CrO5�Ľṹʽ________��
��6��C����ͼ۵��⻯��ΪCH3��ͨ������£�G2+����Һ���ȶ�������CH3�γɵ���λ��Ϊ6��������ȴ���ȶ����ڿ������ױ�����Ϊ[G(CH3)6]3+���÷�Ӧ�����ӷ���ʽ�� ��1 mol [G(CH3)6]3+�����Ӻ���������ĿΪ ��
���𰸡���1����Ar��3d54s1��1�֣����������ڡ���VIII�壨1�֣���2��C��O��N��1�֣���
���Ӽ������Լ���2�֣���3��b��2�֣�����4��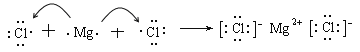
��5��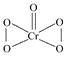 ��6��4[Co(NH3)6]2+��O2��2H2O ==4[Co(NH3)6]3+��4OH��24 NA
��6��4[Co(NH3)6]2+��O2��2H2O ==4[Co(NH3)6]3+��4OH��24 NA
�����������������A��B��C��D��E��F��G����ǰ������Ԫ����ԭ��������������A��������ۺ�����۵ľ���ֵ��ȣ���A�ǵ�IA������A��Ԫ�ء�B�Ļ�̬ԭ����3����ͬ���ܼ��Ҹ��ܼ��е�������ȣ���˵��B��̼Ԫ�أ�����A����Ԫ�ء�D�Ļ�̬ԭ����B�Ļ�̬ԭ�ӵ�δ�ɶԵ�����Ŀ��ͬ����D����Ԫ�أ�����C�ǵ�Ԫ�ء�E�Ļ�̬ԭ��s�ܼ��ĵ���������p�ܼ��ĵ�������ȣ�����E��þԪ�ء�G��3d�����3��δ�ɶԵ��ӣ�˵��G��ԭ��������18+7+2��29����G��Co��F�Ļ�̬ԭ�ӵ�3d�����������4s��������5��������F��ԭ��������18+5+1��24����F�Ǹ�Ԫ�ء���
��1��F��ԭ��������24������ݺ�������Ų����ɿ�֪����̬ԭ�ӵ����Ų�ʽΪ��Ar��3d54s1��Co�����ڱ���λ���ǵ������ڡ���VIII�塣
��2���ǽ�����Խǿ����һ������Խ�����ڵ�Ԫ�ص�2p������Ӵ��ڰ����״̬���ȶ���ǿ����һ�����ܴ�����Ԫ�صģ���B��C��D��ԭ�ӵĵ�һ��������С�����˳��ΪC��O��N��A��C��D�γɵ����ӻ���������Σ����еĻ�ѧ�����������Ӽ������Լ���
��3��a����Ȳ���ӵĽṹʽΪH��C��C��H�����Է����ж�����������������˫��ˮ�ĽṹʽΪH��O��O��H��������ֻ��������a����b��˫��ˮ���Ӽ����������е������Ȳ��b��ȷ��c�����Ǻ����Լ��ͷǼ��Լ�������Ȳ�ǷǼ��Է��ӣ���˫��ˮ�Ǽ��Է��ӣ�c����d�����ߵļ۵���������ȣ����ܻ�Ϊ�ȵ����壬���ӵĿռ乹����ȲΪֱ���Σ�˫��ˮ����ֱ���ͣ�d����e������ԭ��̼ԭ����sp�ӻ�����ԭ����sp3�ӻ���e����ѡb��
��4���Ȼ�þ�Ǻ������Ӽ������ӻ�������γɹ���Ϊ
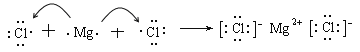 ��
��
��5��F���������Ϊ+6�ۣ�����ԭ�����ֻ���γ�2�����ۼ������CrO5�ĽṹʽΪ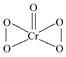 ��
��
��6��C����ͼ۵��⻯��ΪCH3��ͨ������£�G2+����Һ���ȶ�������CH3�γɵ���λ��Ϊ6��������ȴ���ȶ����ڿ������ױ�����Ϊ[G(CH3)6]3+���÷�Ӧ�����ӷ���ʽ��4[Co(NH3)6]2+��O2��2H2O ==4[Co(NH3)6]3+��4OH��������������������1 mol [G(CH3)6]3+�����Ӻ���������ĿΪ24NA��

 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д�����Ŀ����ˮú���ǹ�ҵ�ϳɰ���ԭ����������Ҫ�ɷ���H2��CO��CO2��N2��H2O��g������ˮú���������в���ת��Ϊ�ϳɰ���ԭ�ϡ�
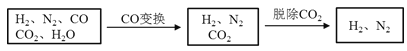
���������գ�
��1����ˮú�������������⡣����ˮú����Ʒͨ��____��Һ�У���д�Լ����ƣ�������_______������֤����������ڡ�
��2����ˮú����ͭ����ʵ��CO�任��CO+H2O![]() CO2+H2
CO2+H2
����ˮú����V(H2):V(CO):V(N2)=38��28��22����CO�任��������У�V(H2):V(N2)=____________��
��3����Һ���շ����ѳ�������̼�ķ���֮һ����֪��
Na2CO3 | K2CO3 | |
20����Һ���Ũ�ȣ�mol/L�� | 2.0 | 8.0 |
��ļ۸�Ԫ/kg�� | 1.25 | 9.80 |
��ѡ��Na2CO3��Һ������Һ�����ŵ���__________��ȱ����____________�����ѡ��K2CO3��Һ������Һ����ʲô�������Խ��ͳɱ���
___________________________________________
д�����ַ����漰�Ļ�ѧ��Ӧ����ʽ��_______________________
��4�������Dzⶨ��ˮú����H2�Լ�CO�����������ʵ�鷽����
ȡһ���������״�����İ�ˮú������������ʵ�鲽��ⶨ����H2�Լ�CO�����������
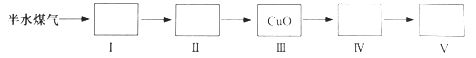
��ѡ�ú��ʵ����Լ��ֱ������������������������С�
����ʵ�鷽���У�������������Ŀ���ǣ�_________________��
����ʵ�鷽���У�����________��ѡ����������������������ȷ����ˮú����H2�����������


