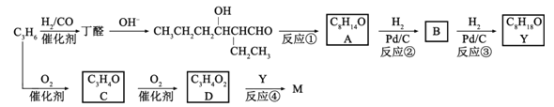
��Ŀ����
����Ŀ��������Ԫ��X��Y��Z��M��ԭ��������������Ԫ��X��һ�ָ�Ӳ�ȵ����DZ�ʯ��Y2+���Ӳ�ṹ������ͬ��Z��������Ϊż����������M����Ϊ����ɫ���壬�ش��������⣺
��1��MԪ��λ�����ڱ��еĵ�______���ڡ�_______�塣
��2��ZԪ����____��������Ȼ���г����Ķ�Ԫ��������____��
��3��X��M�ĵ����ڸ����·�Ӧ�Ļ�ѧ����ʽΪ______���������Ϊֱ���Σ��仯ѧ����__________���ۼ������������������Ǽ���������
��4������Ԫ���е�____�����ں��պ���Ͻ���ϵ��Ʊ����䵥����ϡ���ᷴӦ�Ļ�ѧ����ʽΪ_________��
���𰸡���1������A ��ÿ��1�֣���2�֣�
��2��Si SiO2 ��ÿ��1�֣���2�֣�
��3��C+2S![]() CS2 ���ԣ�ÿ��1�֣���2�֣�
CS2 ���ԣ�ÿ��1�֣���2�֣�
��4��Mg Mg+2HCl==MgCl2+H2�� ��ÿ��1�֣���2�֣�
�����������������������Ԫ��X��Y��Z��M��ԭ��������������Ԫ��X��һ�ָ�Ӳ�ȵ����DZ�ʯ����X��CԪ�أ�Y2+���Ӳ�ṹ������ͬ����Y��MgԪ�أ�Z��������Ϊż����������M����Ϊ����ɫ���壬��Z��SiԪ�أ�M��SԪ�ء���1��MԪ����S����������Ų���2��8��6������λ�����ڱ��еĵ������ڡ���A�壻��2��ZԪ����SiԪ�أ�������Ȼ���г����Ķ�Ԫ��������SiO2����3��X��M�ĵ����ڸ����·�Ӧ����CS2���÷�Ӧ�Ļ�ѧ����ʽΪC+2SCS2���������Ϊֱ���Σ��ṹ��CO2���ƣ������Dz�ͬԪ�ص�ԭ���γɵĹ��ۼ��������仯ѧ�������Թ��ۼ�����4������Ԫ���е�ֻ��Mg�ǽ���Ԫ�أ��ܶȱȽ�С���ƳɵĺϽ�Ӳ�ȴ����Կ����ں��պ���Ͻ���ϵ��Ʊ����ý����DZȽϻ��õĽ��������������ᷢ���û���Ӧ�����������䵥����ϡ���ᷴӦ�Ļ�ѧ����ʽΪMg+2HCl==MgCl2+H2����

 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�����Ŀ����ˮú���ǹ�ҵ�ϳɰ���ԭ����������Ҫ�ɷ���H2��CO��CO2��N2��H2O��g������ˮú���������в���ת��Ϊ�ϳɰ���ԭ�ϡ�
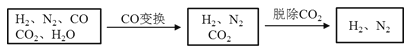
���������գ�
��1����ˮú�������������⡣����ˮú����Ʒͨ��____��Һ�У���д�Լ����ƣ�������_______������֤����������ڡ�
��2����ˮú����ͭ����ʵ��CO�任��CO+H2O![]() CO2+H2
CO2+H2
����ˮú����V(H2):V(CO):V(N2)=38��28��22����CO�任��������У�V(H2):V(N2)=____________��
��3����Һ���շ����ѳ�������̼�ķ���֮һ����֪��
Na2CO3 | K2CO3 | |
20����Һ���Ũ�ȣ�mol/L�� | 2.0 | 8.0 |
��ļ۸�Ԫ/kg�� | 1.25 | 9.80 |
��ѡ��Na2CO3��Һ������Һ�����ŵ���__________��ȱ����____________�����ѡ��K2CO3��Һ������Һ����ʲô�������Խ��ͳɱ���
___________________________________________
д�����ַ����漰�Ļ�ѧ��Ӧ����ʽ��_______________________
��4�������Dzⶨ��ˮú����H2�Լ�CO�����������ʵ�鷽����
ȡһ���������״�����İ�ˮú������������ʵ�鲽��ⶨ����H2�Լ�CO�����������

��ѡ�ú��ʵ����Լ��ֱ������������������������С�
����ʵ�鷽���У�������������Ŀ���ǣ�_________________��
����ʵ�鷽���У�����________��ѡ����������������������ȷ����ˮú����H2�����������