��Ŀ����
����Ŀ����֪ij��84����Һ��ƿ�岿�ֱ�ǩ��ͼ��ʾ���á�84����Һ��ͨ��ϡ��100��(���֮��)
��ʹ�á���ش��������⣺

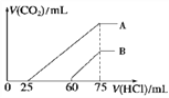
��1���á�84����Һ�������ʵ���Ũ��ԼΪ_____mol��L��1��
��2��ȡ����������ĸ�����Һʱ�������������л�����ȡ����Ķ��ٶ��仯����________(����ĸ)��
A����Һ��NaClO�����ʵ��� B����Һ��Ũ��
C����Һ��NaClO��Ħ������ D����Һ���ܶ�
��3����ͬѧ���ĸá�84����Һ�����䷽������NaClO��������480 mL��NaClO��������Ϊ25%������Һ���ش��������⡣
����ͼ��ʾ�������У���Щ�Dz���Ҫ������������Һ����Ҫ_____________��������

����Ҫ����NaClO���������Ϊ_______ g
��4����84����Һ����ϡ������ʹ�ÿ���ǿ����������ij����С����Ա��98%(�ܶ�Ϊ1.84 g��cm��3)��Ũ��������200 mL 2.3 mol��L��1��ϡ����������ǿ��84����Һ��������������
�������Ƶ�ϡ�����У�H�������ʵ���Ũ��Ϊ________ mol��L��1��
������Ũ��������Ϊ________ mL��
���������Ƶ�ϡ����Ũ��ƫС�������п��ܵ�ԭ���������ȷ����_______��
A������ǰ������ƿ������������ˮ B����ȡŨ����ʱ������Һ��İ�Һ��
C��δ��ȴ������ת��������ƿ���� D������ʱ��������Һ�İ�Һ��
���𰸡�4.0A����������ͷ�ι�149.04.625.0D
��������
��1������c=![]() =1000��1.19��25%/74.5mol��L��1��4.0mol��L��1����2��A�����ʵ����ʵ���Ũ�Ȳ��䣬����c=n/V����������ͬ����ȡ��NaClO�����ʵ�����ͬ����A�������⣻B����ҺΪ��һ���������Һ��Ũ�ȱ��ֲ��䣬��B���������⣻C��NaClO��Ħ����������74.5g��mol��1����C���������⣻D���ܶȲ�������ı仯���仯����D���������⣻��3�����ù�������һ�����ʵ���Ũ�ȣ������Ҫ��������������ƽ��ҩ�ס��ձ�����������500mL ����ƿ����ͷ�ιܣ���Ҫ�IJ����������в������ͽ�ͷ�ιܣ�����Ϊʵ����û��480mL������ƿ�������Ҫ500mL������ƿ�������Ҫ������NaClO������Ϊ500��10��3��4��74.5g=149.0g����4���������Ƶ�������c(H��)=2c(H2SO4)=2��4.3mol��L��1=4.6mol��L��1����Ũ��������ʵ���Ũ��Ϊ1000��98%��1.84/98mol��L��1=18.4mol��L��1��ϡ��ǰ���������ʵ������䣬200��10��3��2.3=V(H2SO4)��10��3��18.4��V(H2SO4)=25.0mL����A������ƿ���Ƿ���ˮ����ʵ������Ӱ�죬��A���������⣻B����ȡŨ����ʱ�����Ӱ�Һ�棬������Һ�����ʵ����ʵ�������Ũ��ƫ�ߣ���B���������⣻C��δ��ȴֱ��ת�Ƶ�����ƿ��Ȼ���ݣ���ȴ�ָ������£�����ƿ����Һ��������٣�������ҺŨ��ƫ�ߣ���C���������⣻D������ʱ�����Ӱ�Һ�棬����ƿ����Һ�����ƫ�ߣ�Ũ��ƫ�ͣ���D�������⡣
=1000��1.19��25%/74.5mol��L��1��4.0mol��L��1����2��A�����ʵ����ʵ���Ũ�Ȳ��䣬����c=n/V����������ͬ����ȡ��NaClO�����ʵ�����ͬ����A�������⣻B����ҺΪ��һ���������Һ��Ũ�ȱ��ֲ��䣬��B���������⣻C��NaClO��Ħ����������74.5g��mol��1����C���������⣻D���ܶȲ�������ı仯���仯����D���������⣻��3�����ù�������һ�����ʵ���Ũ�ȣ������Ҫ��������������ƽ��ҩ�ס��ձ�����������500mL ����ƿ����ͷ�ιܣ���Ҫ�IJ����������в������ͽ�ͷ�ιܣ�����Ϊʵ����û��480mL������ƿ�������Ҫ500mL������ƿ�������Ҫ������NaClO������Ϊ500��10��3��4��74.5g=149.0g����4���������Ƶ�������c(H��)=2c(H2SO4)=2��4.3mol��L��1=4.6mol��L��1����Ũ��������ʵ���Ũ��Ϊ1000��98%��1.84/98mol��L��1=18.4mol��L��1��ϡ��ǰ���������ʵ������䣬200��10��3��2.3=V(H2SO4)��10��3��18.4��V(H2SO4)=25.0mL����A������ƿ���Ƿ���ˮ����ʵ������Ӱ�죬��A���������⣻B����ȡŨ����ʱ�����Ӱ�Һ�棬������Һ�����ʵ����ʵ�������Ũ��ƫ�ߣ���B���������⣻C��δ��ȴֱ��ת�Ƶ�����ƿ��Ȼ���ݣ���ȴ�ָ������£�����ƿ����Һ��������٣�������ҺŨ��ƫ�ߣ���C���������⣻D������ʱ�����Ӱ�Һ�棬����ƿ����Һ�����ƫ�ߣ�Ũ��ƫ�ͣ���D�������⡣

 �¿α�ͬ��ѵ��ϵ�д�
�¿α�ͬ��ѵ��ϵ�д� һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д�����Ŀ��ͼ1��ʾ�ĵ�ѭ������̬ϵͳ����ѭ������Ҫ��ɲ��֣������Ӿ��˵�ѭ���е�����ת����
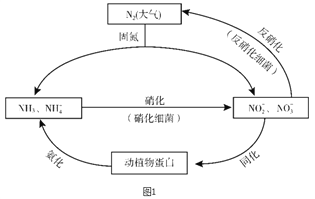
��1�������ͼ�ж�����˵����ȷ����________������ĸ��ţ���
A. �̵������У�N2ֻ��������
B. ������ϸ�������·���������������Ҫ������������
C. �����������������ֲ��˹��̵��Ե�ѭ����ɵ�Ӱ��
D. ͬ�������������У���Ԫ�ؾ�������ת�����л���
��2�����������У�NH3ת����HNO2�ķ�Ӧ�Ļ�ѧ����ʽΪ_______��
��3�������������У�CH3OH����Ϊ��Ӧ�Ļ�ԭ�����뽫�÷�Ӧ�����ӷ���ʽ����������5CH3OH + 6NO3- ![]() N2�� + 4HCO3- +��______+��
N2�� + 4HCO3- +��______+��
��4�������±����ݽ��й��㣬д����ҵ�ϳɰ���Ӧ���Ȼ�ѧ����ʽ��_______��
���ۼ� | N��N | H��H | N��H |
�Ͽ�1mol���ۼ�����������kJ�� | 946 | 436 | 391 |
��5����ⷨ�ϳɰ�����ԭ��ת���ʴ������ߣ��������洫ͳ�Ĺ�ҵ�ϳɰ����ա���ⷨ�ϳɰ�������ԭ����װ����ͼ2��ͼ3��ʾ��
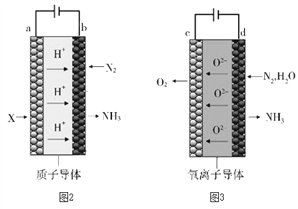
��ͼ2�У�a�缫��ͨ���XΪ_______��
��ͼ3�У�d�缫�ϵĵ缫��ӦʽΪ_______��
����ͼ2��ͼ3װ�õ�ͨ��ʱ����ͬ������ǿ����ȣ����Ч�ʷֱ�Ϊ80%��60%��������װ���в������������ʵ���֮��Ϊ_______��