��Ŀ����
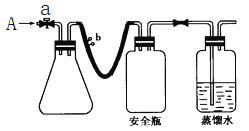
����Ŀ����1��д����ͼ��������������������ƣ�
��___________����___________�� ��___________�� ��___________��
��2�������������У�ʹ��ʱ�������Ƿ�©ˮ����__________����������ţ���
��3����ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص�Ũ�����Լ���ǩ�ϵIJ������ݡ����ø�Ũ��������480 mL 1 mol��L��l��ϡ���ᡣ

�ɹ�ѡ�õ������У� ����ͷ�ιܣ�����ƿ�����ձ�����ҩ�ף�����Ͳ����������ƽ��
��ش��������⣺
������ϡ����ʱ����ȱ�ٵ�������__________��__________��д�������ƣ���
�������㣬����480 mL l mol��L��l��ϡ������Ҫ����Ͳ��ȡ����Ũ��������Ϊ________mL������ȡ�õ�Ũ���Ỻ��ע�뵽ʢˮ���ձ�������Ͳ�ﻹ������Ũ���ᣬ���������ˮϴ�Ӻ�һ����ϴ��Һע���ձ�����ʹ���Ũ��______������ƫ������ƫ����������Ӱ��������
����ת������ƿǰ�ձ���Һ��Ӧ_______�������ʹ���Ũ��____������ƫ������ƫ����������Ӱ��������
������ʱ����ʹ��Һ�İ�Һ����̶�����ƽ�������ӻ�ʹŨ��______��������ƫ������ƫ����������Ӱ��������
���𰸡� Բ����ƿ ������ ��Һ©�� 100ml����ƿ �ۢ� 500ml����ƿ ������ 27.2 ƫ�� �ָ����� ƫ�� ƫ��
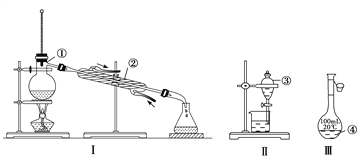
�������������������1������ͼʾд�١�����������������2������ĥ�ڲ�����������ʹ��ʱ�������Ƿ�©ˮ����3���� ��������һ�����ʵ���Ũ�ȵ���Һ���������Ҫ��������������480 mL l mol��L��l��ϡ��������Ҫ500mL����ƿ������ϡ��ǰ���������ʵ������������ҪŨ�������������������ˮϴ����Ͳ��һ����ϴ��Һע���ձ�����ʹ�������ʵ���ƫ��Ũ��������ˮ���ȣ���ֱ��ת������ƿ���������Һ���ƫС���� ����ʱ�����ӿ̶���ʹ��Һ���ƫС��
��������1������ͼʾ�١������������Ʒֱ���Բ����ƿ������������Һ©����100ml����ƿ����2������ĥ�ڲ�����������ʹ��ʱ�������Ƿ�©ˮ��������������������Ҫ�����Ƿ�©ˮ�з�Һ©����100ml����ƿ����3��������һ�����ʵ���Ũ�ȵ���Һ�����У����㡢��ȡ��ϡ�Ͳ���ȴ�����¡���Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ���棬������Ҫ�������н�ͷ�ι����ձ�����Ͳ��500ml����ƿ����������������![]() ,����������98%���ܶ�Ϊ1.84g/cm3��Ũ���ᣬ���ʵ���Ũ����
,����������98%���ܶ�Ϊ1.84g/cm3��Ũ���ᣬ���ʵ���Ũ����![]() ������480 mL l mol��L��l��ϡ��������Ҫ500mL����ƿ������ϡ��ǰ���������ʵ���������
������480 mL l mol��L��l��ϡ��������Ҫ500mL����ƿ������ϡ��ǰ���������ʵ��������� ![]() ��
�� ![]() ��v=0.0272L��������Ҫȡ����Ũ��������Ϊ27.2mL�����������ˮϴ����Ͳ��һ����ϴ��Һע���ձ�����ʹ�������ʵ���ƫ����ҺŨ��ƫ�ߣ���Ũ��������ˮ���ȣ���ֱ��ת������ƿ���������Һ���ƫС����ҺŨ��ƫ�ߣ�������ת������ƿǰ�ձ���Һ��Ӧ��ȴ���������� ����ʱ�����ӿ̶���ʹ��Һ���ƫС����ҺŨ��ƫ����
��v=0.0272L��������Ҫȡ����Ũ��������Ϊ27.2mL�����������ˮϴ����Ͳ��һ����ϴ��Һע���ձ�����ʹ�������ʵ���ƫ����ҺŨ��ƫ�ߣ���Ũ��������ˮ���ȣ���ֱ��ת������ƿ���������Һ���ƫС����ҺŨ��ƫ�ߣ�������ת������ƿǰ�ձ���Һ��Ӧ��ȴ���������� ����ʱ�����ӿ̶���ʹ��Һ���ƫС����ҺŨ��ƫ����

����Ŀ���±��ж�Ӧ��ϵ�������(����)
A | NaCl===Na����Cl���� NH3��H2O | �����ڵ��뷽��ʽ |
B | Ba2����SO42-===BaSO4���� HCO3-��OH��===CO32-��H2O | ���ɱ�ʾһ�෴Ӧ |
C | SO2ʹ���Ը��������Һ��ɫ��SO2ʹ���Է�̪��Һ��ɫ | ������SO2��ͬһ���� |
D | Cl2��2NaOH===NaCl��NaClO��H2O�� 3S��6NaOH===2Na2S��Na2SO3��3H2O | Cl2��S�ڷ�Ӧ�м���������������ԭ�� |
A. A B. B C. C D. D