��Ŀ����
����Ŀ��ʵ��������460mL0.1mol/LNaOH��Һ���ش��������⣺
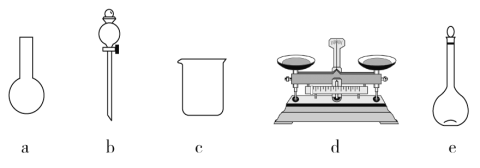
��1����ɱ�ʵ��������������У�������ƽ��ҩ�ס��ձ�������������Ͳ��_____��_____�ȡ�
��2��Ӧ��������ƽ��ȡNaOH���������Ϊ_________��
��3����������������NaOH��ҺŨ��ƫ�ߵ���_________________
A .�������õ�NaOH�������С�ձ����ܽ⣬δ����ȴ����ת�Ƶ�����ƿ�в�����
B .ҡ�Ⱥ���Һ����ڿ̶��ߣ��μ�����ˮ���̶�����ҡ��
C .����ʱ��������ƿ�Ŀ̶���
D������ƿ�ڱڸ���ˮ���δ���ﴦ��
E�� NaOH��������ձ��г���ʱ�����
��4��ȡ���������Ϊ150mL�� A��B ����NaOH ��Һ���ֱ�ͨ��һ������ CO2 ������������Һ�еμ�һ�����ʵ���Ũ�ȵ����� , ���� CO2 �����(��״��)����������������ϵ��ͼ��ʾ��

��B ���߱�����ԭ��Һͨ��CO2��������Һ�����ʵĻ�ѧʽΪ______________��
��A ���߱����������Ũ��Ϊ______mol/L��ͨ���CO2�ڱ�״���µ����Ϊ______mL��
���𰸡� 500mL����ƿ ��ͷ�ι� 2.0g AC Na2CO3 ��NaOH 0.2 224
�����������������ʵ��������460mL0.1mol/LNaOH��Һ����Ҫѡ��500ml����ƿ����500ml��Һ��������Ҫ0.05mol NaOH��������Ϊ2.0g��
��1����ɱ�ʵ��������������У�������ƽ��ҩ�ס��ձ�������������Ͳ��500mL����ƿ����ͷ�ιܵȡ�
��2��Ӧ��������ƽ��ȡNaOH���������Ϊ2.0g��
��3��A .�������õ�NaOH�������С�ձ����ܽ⣬δ����ȴ����ת�Ƶ�����ƿ�в���������������Һ���ƫС��Ũ��ƫ����B .ҡ�Ⱥ���Һ����ڿ̶��ߣ��μ�����ˮ���̶�����ҡ������������Һ���ƫ��Ũ��ƫ�ͣ�C .����ʱ��������ƿ�Ŀ̶�������������Һ���ƫС��Ũ��ƫ�ߣ� D������ƿ�ڱڸ���ˮ���δ���ﴦ������������Һ��Ӱ�죻E�� NaOH��������ձ��г���ʱ��������������NaOH�����տ����е�ˮ�Ͷ�����̼��ƫС����������Һ��Ũ��ƫ�͡������������������NaOH��ҺŨ��ƫ�ߵ���AC ��
��4��ȡ���������Ϊ150ml�� A��B ����NaOH ��Һ����ÿ����Һ�к���0.015molNaOH���ֱ�ͨ��һ������ CO2 ������������Һ�еμ�һ�����ʵ���Ũ�ȵ����� , ����ͨ����ٶ�����̼��������75mL���ᶼǡ�õõ��Ȼ�����Һ�����������غ���������غ��֪��c(HCl)= ![]() 0.2mol/L������Һ�м���������Һ���Ⱥ����ķ�Ӧ����Ϊ��HCl+NaOH=NaCl+H2O����Na2CO3+HCl=NaHCO3+NaCl����NaHCO3+HCl=NaCl+CO2��+H2O����B ���߱�������Ӧ������15mL������75-60������Ӧ�ٺͷ�Ӧ�ڹ�����60mL���ᣬ��Ϊ�������ƺ�̼�����Ʋ��ܴ������棬����ԭ��Һͨ��CO2��������Һ�����ʵĻ�ѧʽΪNa2CO3��NaOH��
0.2mol/L������Һ�м���������Һ���Ⱥ����ķ�Ӧ����Ϊ��HCl+NaOH=NaCl+H2O����Na2CO3+HCl=NaHCO3+NaCl����NaHCO3+HCl=NaCl+CO2��+H2O����B ���߱�������Ӧ������15mL������75-60������Ӧ�ٺͷ�Ӧ�ڹ�����60mL���ᣬ��Ϊ�������ƺ�̼�����Ʋ��ܴ������棬����ԭ��Һͨ��CO2��������Һ�����ʵĻ�ѧʽΪNa2CO3��NaOH��
��A ���߱����������Ũ��Ϊ0.2 mol/L����Ӧ��������������Ϊ��75-25��mL=50mL����n(CO2)=n(HCl )=0.2mol/L![]() 0.05L=0.01mol������ͨ���CO2�ڱ�״���µ����Ϊ224mL��
0.05L=0.01mol������ͨ���CO2�ڱ�״���µ����Ϊ224mL��

 �����Ծ���ĩ���100��ϵ�д�
�����Ծ���ĩ���100��ϵ�д� ˫��ͬ������ѵ��ϵ�д�
˫��ͬ������ѵ��ϵ�д�