��Ŀ����
��1��25��ʱ��ijNaCl��Һ��c(Cl�C)��1��10��4 mol��L�C1�������Һ��c(Na��)��c(OH��)��
��2��25��ʱ����0.1 mol��L�C1NaOH��Һ��0.06 mol��L�C1��H2SO4��Һ��������(���Ի�Ϻ�����ı仯)����������Һ��pH�� ��25��ʱ��pHֵΪ8��NaOH��Һ��pHֵΪ10��NaOH��Һ�������Ϻ���Һ��������Ũ����ӽ� ��
��3��25��ʱ������������Һ�У���pH=0������ ��0.1 mol��L�C1������ ��0.01 mol��L�C1��NaOH��Һ ��pH=11��NaOH��Һ����ˮ��������������Ũ��֮�Ȣ٩U�کU�۩U���ǣ� (����ĸ)
A��1�U10�U100�U1000 B��0�U1�U12�U11
C��14�U13�U12�U11 D��14�U13�U2�U3
��4��ij�¶�(t��)ʱ�����0.01mol��L��1 ��NaOH��Һ��pH��11��
������¶���ˮ��Kw�� ��
���ڸ��¶��²��ij��ҺpH��3�������Һ��c(H��)��c(OH�� )��________��
�۸��¶��½�pH=2�������pH=11������������Һ�������ϣ�pH=______________
��5�� ��ˮ��c(H+)=5.0��10�C7 mol��L�C1�����ʱ��ˮ�е�c(OH�C) = �����¶Ȳ��䣬����ϡ����ʹc(H+)=5.0��10�C3 mol��L�C1����c(OH�C) = ���ڸ��¶�ʱ����ˮ�е���NaOH��Һ����Һ�е�c(OH�C)=5.0��10�C2 mol��L�C1������Һ��c(H+)= ��
��1��1000:1 ��2��2 2��10�C2 ��3�� A ��4��10�C13 107:1 6.5
��5��5.0��10�C7 mol��L�C1 5.0��10�C11 mol��L�C1 5.0��10�C12 mol��L�C1
��������
�����������1�� c(Na+)��c(Cl�C)��1��10��4
mol��L�C1��c(OH�C)��1��10��7mol��L�C1��c(Na��)��c(OH��)��1��10��4��1��10��7��1000:1����2����NaOH��Һ��H2SO4��Һ�������ΪV�������ڷ�Ӧ��H2SO4����������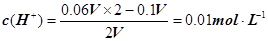 ����pH=�Clg0.01=2��pHֵΪ8��NaOH��Һ��c(OH��)=10��6 mol��L�C1��pHֵΪ10��NaOH��Һ��c(OH��)=10��4
mol��L�C1���������Ϻ�Ļ����Һ��
����pH=�Clg0.01=2��pHֵΪ8��NaOH��Һ��c(OH��)=10��6 mol��L�C1��pHֵΪ10��NaOH��Һ��c(OH��)=10��4
mol��L�C1���������Ϻ�Ļ����Һ��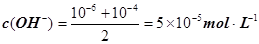 ��������Ũ��Ϊ
��������Ũ��Ϊ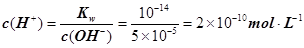 ����3�� ��10�C14 mol��L�C1����10�C13 mol��L�C1����10�C12 mol��L�C1����10�C11 mol��L�C1������ˮ��������������Ũ��֮�Ȣ٩U�کU�۩U��=1�U10�U100�U1000����ΪA����4��
����c(OH�C)=10�C2 mol��L�C1��c(H+)=10�C11 mol��L�C1����Kw= c(H+)��c(OH�C)=10�C13������pH=3���c(H+)=10�C3 mol��L�C1����Kw= c(H+)��c(OH�C)=10�C13���c(OH�C)=10�C10 mol��L�C1�����Ը���Һ��c(H��)��c(OH��)��10�C3��10�C10��107��1���������е�c(H+)=10�C2 mol��L�C1������������Һ��c(OH�C)=10�C2 mol��L�C1������Һ��������ʱ�����ǡ����ȫ�кͣ���Һ�����ԣ�c(H+)=
����3�� ��10�C14 mol��L�C1����10�C13 mol��L�C1����10�C12 mol��L�C1����10�C11 mol��L�C1������ˮ��������������Ũ��֮�Ȣ٩U�کU�۩U��=1�U10�U100�U1000����ΪA����4��
����c(OH�C)=10�C2 mol��L�C1��c(H+)=10�C11 mol��L�C1����Kw= c(H+)��c(OH�C)=10�C13������pH=3���c(H+)=10�C3 mol��L�C1����Kw= c(H+)��c(OH�C)=10�C13���c(OH�C)=10�C10 mol��L�C1�����Ը���Һ��c(H��)��c(OH��)��10�C3��10�C10��107��1���������е�c(H+)=10�C2 mol��L�C1������������Һ��c(OH�C)=10�C2 mol��L�C1������Һ��������ʱ�����ǡ����ȫ�кͣ���Һ�����ԣ�c(H+)=
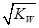 = 10�C6.5
mol��L�C1����pH=6.5����5��ˮ�����������Ũ�����ǵ�������������������Ũ�ȣ�����c(OH�C)=c(H+)=5.0��10�C7 mol��L�C1�����¶���Kw= c(H+)��c(OH�C)= 5.0��10�C7��5.0��10�C7 =2.5��10�C13����c(H+)=5.0��10�C3 mol��L�C1����c(OH�C)
= Kw/c(OH�C)= 5.0��10�C11 mol��L�C1����c(OH�C)=5.0��10�C2
mol��L�C1������Һ��c(H+)= Kw/c(H+)= 5.0��10�C12 mol��L�C1��
= 10�C6.5
mol��L�C1����pH=6.5����5��ˮ�����������Ũ�����ǵ�������������������Ũ�ȣ�����c(OH�C)=c(H+)=5.0��10�C7 mol��L�C1�����¶���Kw= c(H+)��c(OH�C)= 5.0��10�C7��5.0��10�C7 =2.5��10�C13����c(H+)=5.0��10�C3 mol��L�C1����c(OH�C)
= Kw/c(OH�C)= 5.0��10�C11 mol��L�C1����c(OH�C)=5.0��10�C2
mol��L�C1������Һ��c(H+)= Kw/c(H+)= 5.0��10�C12 mol��L�C1��
���㣺������Һ������Ũ�ȼ��㡢pH���㡢ˮ�����ӻ��ļ��㡣

| A���κ�Ũ����Һ������Զ�����pH��ֽ�ⶨ | B��ij�¶��£�pH=6.2�Ĵ�ˮ������ | C��25��ʱ��pH=1�Ĵ�����Һ��c��H+����pH=2��������Һ��c��H+����10�� | D��25��ʱ��pH=12��NaOH��Һ�е�n��OH-��=10-2mol |
 ˮ�ĵ���ƽ��������ͼ��ʾ��
ˮ�ĵ���ƽ��������ͼ��ʾ�� ˮ�ĵ���ƽ��������ͼ��ʾ��?
ˮ�ĵ���ƽ��������ͼ��ʾ��?