��Ŀ����
����Ŀ����������Ϊԭ�ϣ�ͨ��̼�Ȼ�ԭ���ɺϳɵ�������AlN����ͨ����ⷨ����ȡ�����ش��������⣺
��1����֪��2Al2O3(s)=4Al(g)��3O2(g) ��H1=3351kJ��mol��1
2C(s)��O2(g)=2CO(g) ��H2=-221kJ��mol��1
2Al(g)��N2(g)=2AlN(s) ��H3=-318kJ��mol��1
̼�Ȼ�ԭAl2O3�ϳ�AlN�����Ȼ�ѧ����ʽ��___��
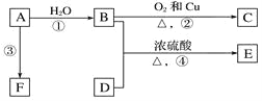
��2����ҵ���õ�������������ķ�������ȡ���������������������۵�ܸߣ�Լ2045�棩����ʵ�������У�ͨ���������ۼ�����ʯ��Na3AlF6����1000�����ҾͿ��Եõ������塣
��ͼ�ǵ��۵�ʾ��ͼ��
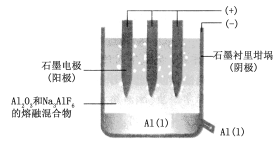
��д�����ʱ�����ĵ缫��Ӧʽ��___��
�ڵ����������ɵ�����ȫ����ʯī�缫��Ӧ����CO��CO2���塣��ˣ���Ҫ���ϲ���ʯī�缫����ҵ�����У�ÿ����9����������ʧ5.4��ʯī��ÿ����9����ת�Ƶ��ӵ����ʵ���Ϊ___mol�����ɵĶ�����̼�����ʵ���Ϊ___mol��
��3�������ڵ綯��������������ȼ�ϵ�أ�ͨ����NaCl��Һ��NaOH��ҺΪ�������Һ�������Ͻ�Ϊ������
����NaCl��ҺΪ�������Һʱ��������ӦʽΪ___��
����NaOH��ҺΪ�������Һʱ��������ӦʽΪ___��
���𰸡�3C(s)+Al2O3(s)+ N2(g)=2AlN(s)+3CO(g) ��H=+1026kJ��mol��1 2O2--4e-= O2�� 106 5��104 O2+2H2O+4e=4OH Al+4OH3e=AlO2+2H2O
��������
��1������������ʽ�ֱ���б�ţ���2Al2O3(s)=4Al(g)��3O2(g)��H1=3351kJ��mol��1����2C(s)��O2(g)=2CO(g)��H2=-221kJ��mol��1����2Al(g)��N2(g)=2AlN(s)��H3=-318kJ��mol��1��![]() ����+
����+![]() ����+�ۿɵõ�̼�Ȼ�ԭAl2O3�ϳ�AlN�ķ�Ӧ����ʽΪ3C+Al2O3+ N2=2AlN+3CO�����ݸ�˹����
����+�ۿɵõ�̼�Ȼ�ԭAl2O3�ϳ�AlN�ķ�Ӧ����ʽΪ3C+Al2O3+ N2=2AlN+3CO�����ݸ�˹����![]() �������Ȼ�ѧ����ʽ��3C(s)+Al2O3(s)+ N2(g)=2AlN(s)+3CO(g) ��H=+1026kJ��mol��1���ʴ�Ϊ��3C(s)+Al2O3(s)+ N2(g)=2AlN(s)+3CO(g) ��H=+1026kJ��mol��1��
�������Ȼ�ѧ����ʽ��3C(s)+Al2O3(s)+ N2(g)=2AlN(s)+3CO(g) ��H=+1026kJ��mol��1���ʴ�Ϊ��3C(s)+Al2O3(s)+ N2(g)=2AlN(s)+3CO(g) ��H=+1026kJ��mol��1��
��2���ٵ�����ڵ�������ʱ�����������ӷŵ磬�缫��ӦʽΪ2O2--4e-= O2�����ʴ�Ϊ��2O2--4e-= O2����
��ÿ����1molAlת�Ƶ���3mol����ÿ����9����ת�Ƶ��ӵ����ʵ���Ϊn= ![]() ��5.4��ʯī������Ϊ
��5.4��ʯī������Ϊ![]() ��ʯī�缫��Ӧ����CO��CO2���壬�����ɶ�����̼�����ʵ���Ϊx���ɵ����غ��֪��
��ʯī�缫��Ӧ����CO��CO2���壬�����ɶ�����̼�����ʵ���Ϊx���ɵ����غ��֪��![]() �����x=5��104mol���ʴ�Ϊ��106��5��104��
�����x=5��104mol���ʴ�Ϊ��106��5��104��
��3������NaCl��ҺΪ�������Һʱ�����������ŵ磬��������ӦʽΪO2+2H2O+4e=4OH���ʴ�Ϊ��O2+2H2O+4e=4OH��
����NaOH��ҺΪ�������Һʱ���������ŵ�ת��Ϊƫ��������缫��ӦʽΪAl+4OH3e=AlO2+2H2O���ʴ�Ϊ��Al+4OH3e=AlO2+2H2O��