��Ŀ����
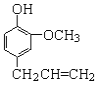
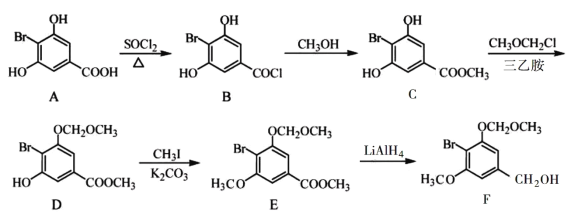
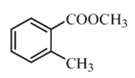
����Ŀ����֪�л���A��B��C��D��E��F������ת����ϵ��A�Ƿ�����Ϊ28������ϩ����������Ǻ���һ������ʯ�ͻ�������ˮƽ�ı�־��D��ʳ����Ҫ�ɷ֣�E�Dz�����ˮ�Ҿ�����ζ����ɫҺ�壬��Է���������C��2����F��һ�ָ߷��ӻ���������ͼ��ϵ�ش����⣺
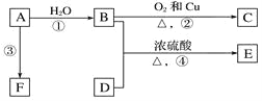
(1)д��B��D�й����ŵ����ƣ�B_____��D_____��
(2)д�����з�Ӧ�Ļ�ѧ����ʽ��
��_____���÷�Ӧ������_____��
��_____���÷�Ӧ������_____��
��_____��
(3)ʵ��������ͼ��װ���Ʊ�E���Թ�A��Ũ�����������______��B�е��Լ���______�����ڸ�ʵ������˵������ȷ����______��
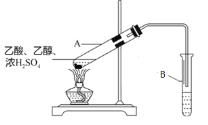
�ټ����Թ�A�������Լӿ췴Ӧ���ʣ�����������E���ٽ���Ӧ����
�ڴ��Թ�A��������������ֻ�в���E
����B����״Һ���䱡��Ҫ�Dz���E�ܽ�Լ�B��
��B�е���Ӧ�ò��뵽Һ������
���𰸡��ǻ� �Ȼ� CH2=CH2+H2O![]() CH3CH2OH �ӳɷ�Ӧ 2CH3CH2OH+O2
CH3CH2OH �ӳɷ�Ӧ 2CH3CH2OH+O2![]() 2CHCHO+2H2O ������Ӧ CH3COOH+CH3CH2OH
2CHCHO+2H2O ������Ӧ CH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O ��������ˮ�� ����̼������Һ �ڢۢ�
CH3COOCH2CH3+H2O ��������ˮ�� ����̼������Һ �ڢۢ�
��������
A�Ƿ�����Ϊ28������ϩ����������Ǻ���һ������ʯ�ͻ�������ˮƽ�ı�־������AΪCH2=CH2��A��ˮ�����ӳɷ�Ӧ���ɵ�BΪCH3CH2OH��B������������CΪCH3CHO��D��ʳ����Ҫ�ɷ֣�����DΪCH3COOH��D��B����������Ӧ����EΪCH3COOCH2CH3�����������ĶԷ�������Ϊ88��CHCHO����Է�������Ϊ44���������⣻FΪһ�ָ߷��ӻ����ӦΪ��ϩ�����ۺϷ�Ӧ���ɵľ���ϩ��
(1)BΪCH3CH2OH���������Ϊ�ǻ���DΪCH3COOH���������Ϊ�Ȼ���
(2)��Ӧ��Ϊ��ϩ��ˮ�ļӳɷ�Ӧ����ѧ����ʽΪCH2=CH2+H2O![]() CH3CH2OH����Ӧ����Ϊ�ӳɷ�Ӧ��
CH3CH2OH����Ӧ����Ϊ�ӳɷ�Ӧ��
��Ӧ��Ϊ�Ҵ��Ĵ���������ѧ����ʽΪ2CH3CH2OH+O2![]() 2CHCHO+2H2O����Ӧ����Ϊ������Ӧ��
2CHCHO+2H2O����Ӧ����Ϊ������Ӧ��
��Ӧ��Ϊ�Ҵ��������������Ӧ����ѧ����ʽΪCH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
(3)����������������Ӧ��Ũ���������������������ˮ����B��Ϊ����̼������Һ���������ջӷ���������Ҵ������������������ܽ�ȣ�������Һ�ֲ㣻
�ټ��ȿ��Լӿ췴Ӧ���ʣ�����������ٽ���Ӧ�����ƶ����ʢ���ȷ��
�ڴ��Թ�A�������������ʣ�������������֮�⣬����������Ҵ����ʢڴ���
�����������ڱ���̼������Һ�е��ܽ�Ⱥ�С����B����״Һ���䱡��Ҫ�ǻ��е�������Ҵ���̼������Һ���գ��ʢ۴���
�ܴ��Թ�A�г����������к���������Ҵ������߱�̼������Һ���գ�����B�е��ܲ��뵽Һ�����»ᷢ���������ʢܴ���
��������ѡ�ڢۢܡ�

 ��ĩ100�ִ��غ�������ϵ�д�
��ĩ100�ִ��غ�������ϵ�д� Сѧ�������Ծ�ϵ�д�
Сѧ�������Ծ�ϵ�д�����Ŀ���о���ѧ��Ӧ�������仯�����ʱ仯���о���ѧ��Ӧ����Ҫ�Ƕȡ�
��1����ѧ��Ӧ�������仯����Ҫԭ���ǾɵĻ�ѧ�����ѻ�_____�������µĻ�ѧ���γɻ�_____�������������ų���������������
��2�������ȷ���ұ�������䷴ӦΪ��Fe2O3��2Al![]() 2Fe��Al2O3�����ڷ��ȷ�Ӧ����Ӧ���������______��������������������������������������������ڸ÷�Ӧ�У���������1molAl���������Ͽ�����Fe�����ʵ���Ϊ_____mol��
2Fe��Al2O3�����ڷ��ȷ�Ӧ����Ӧ���������______��������������������������������������������ڸ÷�Ӧ�У���������1molAl���������Ͽ�����Fe�����ʵ���Ϊ_____mol��
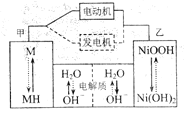
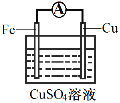
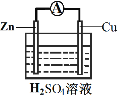
��3��Ϊ̽����Ӧ�����е������仯��ijС��ͬѧ����ͼװ�ý���ʵ�顣
|
|
װ�â� | װ�â� |
��װ�â��У�Fe��CuSO4��Һ��Ӧ�����ӷ���ʽ��_____��
��װ�â��У������ĵ缫��ӦʽΪ______��
�۹���װ�â�����������ȷ����______������ĸ����
a.H+��Cu���汻��ԭ����������
b.������ZnƬ����������CuƬ
c.���Ӵ�ZnƬ����������CuƬ
d.Zn��Cu�Ķ��ǵ缫���ϣ�Ҳ������缫��Ӧ
��4��ij��ȤС�齫��ȥ����Ĥ��þ��Ͷ�뵽����ϡ�����н���ʵ�飬ʵ���������IJ������ʱ仯�����ͼ������ʾ���Ը����ߵĽ�������ȷ����_____��
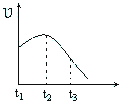
A.��t1��t2��ԭ����þ����ķ�Ӧ�Ƿ��ȷ�Ӧ����ϵ�¶�����
B.��t1��t2��ԭ��ˮ��������ʹ���Ũ������
C.��t2��t3��ԭ�������ŷ�Ӧ�Ľ���þ���������½�
D.��t2��t3��ԭ�������ŷ�Ӧ�Ľ��У�H+��Ũ�����½�