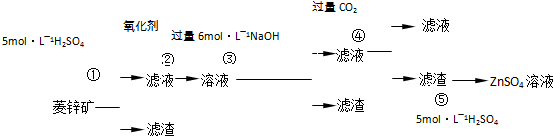
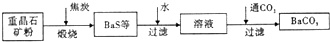
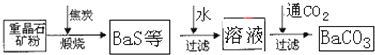
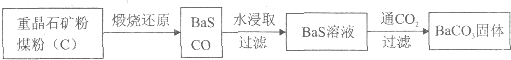
��Ŀ����
�ؾ�ʯ��BaSO4��������ˮ��Ҫת����BaCO3���Ʊ��������Ρ���ҵ��һ����ø������ջ�ԭ����ʵ���ҿ��Բ��ó���ת������
��������ջ�ԭ��

��1�����ջ�ԭ���Ȼ�ѧ����ʽΪ��
�йص����ݣ�Ba(s)+S(s)+2O2(g)=BaSO4(s)����H= ��1473.2 kJ?mol��1
C(s)+![]() O2(g)=CO(g)����H= ��110.5 kJ?mol��1
O2(g)=CO(g)����H= ��110.5 kJ?mol��1
Ba(s)+S(s)=BaS(s)����H= ��460kJ?mol��1
��2��������BaS��ˮ��Һ�ʼ��ԣ�ԭ���ǣ������ӷ���ʽ��ʾ��:
.
�����ת����
��BaSO4�����м��뱥��Na2CO3��Һ����ֽ��裬��ȥ�ϲ���Һ����˴�����Σ�ֱ��BaSO4ȫ��ת��ΪBaCO3��BaSO4(s)+CO32-(aq)BaCO3(s)+SO42-(aq)��ƽ�ⳣ��K=0.042��
![]() ��3������0.20 mol BaSO4��ÿ����1.00 L 2.0mol?L��1����Na2CO3��Һ�������ٶ�BaSO4��ȫ��ת����������Ҫ�������ٴΣ���д��������̣�
��3������0.20 mol BaSO4��ÿ����1.00 L 2.0mol?L��1����Na2CO3��Һ�������ٶ�BaSO4��ȫ��ת����������Ҫ�������ٴΣ���д��������̣�![]()
![]() ��4���ӡ���ɫ��ѧ���Ƕȷ������ñ���Na2CO3��Һ����ת�������ŵ���
��4���ӡ���ɫ��ѧ���Ƕȷ������ñ���Na2CO3��Һ����ת�������ŵ���
��1��BaSO4��s����4C��s��= BaS��s����4CO��g�� ��H=+571.2 kJ•mol-1 ��2�֣�
��2��S2- + H2O ![]() HS�� + OH�������֣�
HS�� + OH�������֣�
��3��������� ����ÿ����1.00L 2.0 mol•L��1����Na2CO3��Һ�ܴ���xmol BaSO4
BaSO4��CO32-��BaCO3��SO42-
(2.0-x) mol•L��1 x mol•L��1
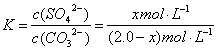 == 0.042 (����)
== 0.042 (����)
X = 0.081��0.084Ҳ���֣� (1��)��
�������� = 0.2mol/0.081mol=2.5(1��)��2.38Ҳ���֣���
�����ٴ���3�� (1��)
��4�����ܣ����� ����1�֣���2�֡������𰸾Ϳɵ÷֣�

 �����ܿ����ϵ�д�
�����ܿ����ϵ�д�