��Ŀ����
��ҵ�Ϻϳɰ����ȷ�Ӧ����ʽ���£�N2(g)+3H2(g) 2NH3(g) ��H����92 kJ/mol
2NH3(g) ��H����92 kJ/mol
��1������֪�ƻ�1mol ����H��H�����ֱ������յ�����Ϊ946 kJ��436 kJ����Ͽ�1molN��H�����յ�����Ϊ kJ��
����H��H�����ֱ������յ�����Ϊ946 kJ��436 kJ����Ͽ�1molN��H�����յ�����Ϊ kJ��
��2���ں��º�ѹ�������£���2mol N2��6molH2ͨ��һ�ݻ��ɱ�������з�Ӧ���ﵽƽ�����������Ϊ��Ӧǰ��75%����ù����ͷŵ�����Ϊ kJ��������ת����Ϊ ��ƽ�����ռ���������������Ϊ ��
��3������1mol N2��1molH2ͨ��������ͬ������ܱ����������У������������¶Ⱥ�������䣬�����������¶Ⱥ�ѹǿ���䣬����һ��ʱ������������ﵽƽ��״̬��
�ٽ���ƽ�������ʱ�䣺�� �ң������,������������
�ڴﵽƽ������������������ �ң������,������������
 2NH3(g) ��H����92 kJ/mol
2NH3(g) ��H����92 kJ/mol��1������֪�ƻ�1mol
 ����H��H�����ֱ������յ�����Ϊ946 kJ��436 kJ����Ͽ�1molN��H�����յ�����Ϊ kJ��
����H��H�����ֱ������յ�����Ϊ946 kJ��436 kJ����Ͽ�1molN��H�����յ�����Ϊ kJ����2���ں��º�ѹ�������£���2mol N2��6molH2ͨ��һ�ݻ��ɱ�������з�Ӧ���ﵽƽ�����������Ϊ��Ӧǰ��75%����ù����ͷŵ�����Ϊ kJ��������ת����Ϊ ��ƽ�����ռ���������������Ϊ ��
��3������1mol N2��1molH2ͨ��������ͬ������ܱ����������У������������¶Ⱥ�������䣬�����������¶Ⱥ�ѹǿ���䣬����һ��ʱ������������ﵽƽ��״̬��
�ٽ���ƽ�������ʱ�䣺�� �ң������,������������
�ڴﵽƽ������������������ �ң������,������������
��1�� 391��2�� 92��50%��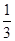 ��33.3% ��3���� �� �� ��
��33.3% ��3���� �� �� ��
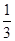 ��33.3% ��3���� �� �� ��
��33.3% ��3���� �� �� �������������1����Ӧ�ȵ��ڷ�Ӧ����ܼ��ܼ�ȥ��������ܼ��ܣ���N��H����Ϊ xkJ/mol����945.6kJ/mol��3��436 kJ/mol��6��x kJ/mol����92.2kJ/mol�����x��391��
��2�� N2(g)+3H2(g)
 2NH3(g)
2NH3(g)��ʼ����mol�� 2 6 0
ת������mol�� x 3x 2x
ƽ������mol�� 2��x 6��3x 2x
����ݴﵽƽ�����������Ϊ��Ӧǰ��75%��֪
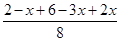 ��0.75
��0.75���x��1
���Ըù����ͷŵ�����Ϊ92kJ
������ת����Ϊ
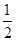 ��100%��50%
��100%��50%ƽ�����ռ���������������Ϊ
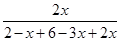 =
=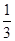
��3���ٸ��ݷ���ʽN2(g)+3H2(g)
 2NH3(g)��֪������Ӧ�������С�Ŀ��淴Ӧ��������������ݻ����䣬��ѹǿ���ͣ���˵���ڷ�Ӧ�������������е�ѹǿʼ�մ��ڼ������е�ѹǿ��ѹǿ��Ӧ���ʿ죬����ƽ���ʱ���٣�������ƽ�������ʱ�䣺�ף��ҡ�
2NH3(g)��֪������Ӧ�������С�Ŀ��淴Ӧ��������������ݻ����䣬��ѹǿ���ͣ���˵���ڷ�Ӧ�������������е�ѹǿʼ�մ��ڼ������е�ѹǿ��ѹǿ��Ӧ���ʿ죬����ƽ���ʱ���٣�������ƽ�������ʱ�䣺�ף��ҡ�������Ӧ�������С�Ŀ��淴Ӧ�����ѹǿ��������ƽ��������Ӧ�����ƶ�����������������������Դﵽƽ�����������������ף��ҡ�

��ϰ��ϵ�д�
�����Ŀ
 O2(g)=ZnO(s)����H1����351.1 kJ��mol��1
O2(g)=ZnO(s)����H1����351.1 kJ��mol��1 3N2��2X��4H2O
3N2��2X��4H2O  O2(g)=H2O(l) ��H����285.8 kJ��mol��1
O2(g)=H2O(l) ��H����285.8 kJ��mol��1

