��Ŀ����
16��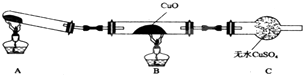 ijУ�о���ѧϰС���ͬѧѧϰ�굪���й����ʵ�����֮�Ե�Ԫ�ص��⻯��NH3���ʵ�̽����
ijУ�о���ѧϰС���ͬѧѧϰ�굪���й����ʵ�����֮�Ե�Ԫ�ص��⻯��NH3���ʵ�̽������1��ʵ������ȡ�����Ļ�ѧ����ʽΪ2NH4Cl+Ca��OH��2 $\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
��2��ijͬѧģ���ű���ʳ��ˮ�ռ������ķ����������ű����Ȼ����Һ�ķ����ռ�����������Ϊ���ܷ�ﵽĿ�ģ�����ܡ����������ǰ�����������ˮ���Ȼ�臨�����ˮ�е��ܽ�Ӱ�첻��
��3����С���ͬѧ�������ͼ��ʾ��ʵ��װ�ã��гּ�β������װ��δ��������̽�������Ļ�ԭ�ԣ�
�ٸ�װ�����������һ��ȱ�ݣ�Ϊ��֤ʵ������ȷ�ԣ��Ը�װ�õĸĽ���ʩ����װ��A��B֮������װ�м�ʯ�ҵ�U�ܣ�
�����øĽ����װ�ý���ʵ�飬CuO��Ϊ��ɫ���ʣ���ˮCuSO4������ͬʱ����һ������Ⱦ�����壮������CuO��Ӧ�Ļ�ѧ����ʽΪ3CuO+2NH3$\frac{\underline{\;\;��\;\;}}{\;}$3Cu+N2+3H2O��
����ͬѧ��ΪNH3��CuO��Ӧ���ɵĺ�ɫ�����п��ܺ�Cu2O��Cu2O��������Һ��Cu+�绯����Cu��Cu2+�������һ����ʵ�����ú�ɫ�������Ƿ���Cu2Oȡ������Ʒ������ϡ���ᣬ����Һ������ɫ��˵������Cu2O�������У�
���� ��1���Ȼ�������������ڼ������������ɰ������Ȼ��ơ�ˮ��
��2��������������ˮ���Ȼ�臨�����ˮ�е��ܽ�Ӱ�첻��
��3�����Ȼ�狀���ʯ�ҷ�Ӧ���ɰ�����ˮ��������CuO��ӦǰӦ�ȸ��
�ڰ�����CuO��Ӧ����ͭ��������ˮ��
������������ͭ������Һ�з�������������ԭ��Ӧ����ͭ��ͭ���ӣ���Һ��ɫ֤��������ͭ�Ĵ��ڣ�
��� �⣺��1���Ȼ�������������ڼ������������ɰ������Ȼ��ơ�ˮ������ʽΪ2NH4Cl+Ca��OH��2 $\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
�ʴ�Ϊ��2NH4Cl+Ca��OH��2 $\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
��2��������������ˮ���Ȼ�臨�����ˮ�е��ܽ�Ӱ�첻�����Բ��������ű����Ȼ����Һ�ķ����ռ�������
�ʴ�Ϊ��������������ˮ���Ȼ�臨�����ˮ�е��ܽ�Ӱ�첻��
��3�����Ȼ�狀���ʯ�ҷ�Ӧ���ɰ�����ˮ��������CuO��ӦǰӦ�ȸ���ʴ�Ϊ����װ��A��B֮������װ�м�ʯ�ҵ�U�ܣ�
�ڰ�����CuO��Ӧ����ͭ��������ˮ����ѧ����ʽ��3CuO+2NH3$\frac{\underline{\;\;��\;\;}}{\;}$3Cu+N2+3H2O���ʴ�Ϊ��3CuO+2NH3$\frac{\underline{\;\;��\;\;}}{\;}$3Cu+N2+3H2O��
��Cu20��һ�ּ����������������Һ��Cu+����Cu+Cu2+���ݴ˷�Ӧ���ʵ����֤�Ƿ���������ͭ������Ϊ��ȡ������Ʒ������ϡ���ᣬ����Һ������ɫ��˵������Cu2O�������У��ʴ�Ϊ��ȡ������Ʒ������ϡ���ᣬ����Һ������ɫ��˵������Cu2O�������У�
���� ���⿼���˰���ʵ�����Ʊ�������������������֤ʵ��������ڷ����жϣ���Ϥ�Ʊ�ԭ���ǽ���ؼ���ע��ʵ����Ƶĺ����ԣ���Ŀ�ѶȲ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���1��Sλ��Ԫ�����ڱ��������ڵ�VIA�壻Fe�Ļ�̬ԭ���������2�����ӣ�
��2���á�����������գ�
| ��һ������ | ���Ӱ뾶 | �۵� | ���� |
| N��O | O2-��Al3+ | KCl����� | H2SO4��HClO4 |
��4����KAlO2��Һ�в���ͨ��NO2���壬��Ӧ������Ϊ�����ɰ�ɫ������Ȼ���ɫ���������ܽ⣬�����漰������ԭ��Ӧ�����ӷ���ʽΪ3NO2+H2O�T2H++2NO3-+NO���˷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ1��2��
 ȼú�����к��д����ĵ��������NOx��������ֱ���ŷŵ������У��ɲ������´�ʩ��ȼú�������д�����
ȼú�����к��д����ĵ��������NOx��������ֱ���ŷŵ������У��ɲ������´�ʩ��ȼú�������д�������CH4��ԭ����CH4��g��+4NO2��g��=4NO��g��+CO2��g��+2H2O��g����H1=akJ•mol-1
CH4��g��+4NO��g��=2N2��g��+CO2��g��+2H2O��g����H2=bkJ•mol-1����ֱ�ӽ�NO2��ԭΪN2���Ȼ�ѧ����ʽΪCH4��g��+2NO2��g��=N2��g��+CO2��g��+2H2O��g����H=0.5��a+b��kJ/mol��
CH4��g��+4NO2��g��=4NO��g��+CO2��g��+2H2O��g����H1=akJ•mol-1
���¶�T1��T2ʱ���ֱ�0.50molCH4��1.2molNO2�������Ϊ1L���ܱ������У����n��CH4����ʱ��仯�������±���
| �¶� | ʱ��/min n/mol | 0 | 10 | 20 | 40 | 50 |
| T1 | n��CH4�� | 0.50 | 0.35 | 0.25 | 0.10 | 0.10 |
| T2 | n��CH4�� | 0.50 | 0.30 | 0.18 | 0.15 | 0.15 |
��2��T1�� T2�����=�������� a��0�����=������
��3����T1ʱ��0��10min����NO2��ת������50%�������NO2��ת����ͬʱ�ӿ췴Ӧ���ʣ��ɲ�ȡ�Ĵ�ʩ��B
A����С������� B������CH4Ũ�� C�������¶� D���������
�� NOxҲ������NH3��ԭ��ȥ�����÷�Ӧ6NO2+8NH3$?_{����}^{����}$7N2+12H2OҲ�ɴ���NO2������������ͻ�ԭ�����������Ϊ14gʱ��ת�Ƶ��ӵ����ʵ���Ϊ12mol��
| A�� | +6 | B�� | +3 | C�� | +4 | D�� | +2 |
| A�� | SO42һ | B�� | Cu2+ | C�� | Ag+ | D�� | NO${\;}_{3}^{-}$ |
| A�� | Na2CO3��Һ | B�� | Na2SiO3��Һ | C�� | NaOH��Һ | D�� | NaHSO3��Һ�� |
| A�� | FeCl2 | B�� | Fe3O4 | C�� | Fe��OH��3 | D�� | Fe2��SO4��3 |
| A�� | һ��������Ba2+��NH4+���ܴ��� | B�� | CO32-һ������ | ||
| C�� | Na+һ������ | D�� | һ��������Cl- |

| A�� | t1minʱ�����淴Ӧ������� | |
| B�� | X���߱�ʾNH3�����ʵ�����ʱ��仯�Ĺ�ϵ | |
| C�� | 0��8 min��H2��ƽ����Ӧ����v��H2��=0.75 mol•L-1•min-1 | |
| D�� | 10��12 min��N2��ƽ����Ӧ����Ϊv��N2��=0.25mol•L-1•min-1 |