��Ŀ����
��10�֣����û�ѧԭ�����ԶԹ����ŷŵķ�ˮ������Ч���ͺ���������
��1��Ⱦ�Ϲ�ҵ�ŷŵķ�ˮ�к��д����ж���NO2-�������ڼ��������¼������۳�ȥ(���ȴ�����ķ�ˮ�������ʹʪ���ɫʯ����ֽ����������)����ȥNO2-���ӵ����ӷ���ʽ��
�� ��
��2����ˮ�е�N��PԪ�������ˮ�帻Ӫ��������Ҫ���أ�ũҩ���ŷŵķ�ˮ�г����н϶��NH4+��PO43-��һ�����ͨ�����ַ��������ȥ��
�ٷ���һ����Ca(OH)2��CaOͶ�ӵ��������ķ�ˮ�У���������ƣ��Ӷ����л��ա���������ķ�ˮ��c��Ca2+��=2��10-7mol/Lʱ����Һ��c��PO43-��= ��
(��֪����ʱ����SP��Ca3��PO4��2��=2��10-33)
�ڷ��������ڷ�ˮ�м���þ��ҵ��ˮ���Ϳ������ɸ�Ʒλ����ʯ�������ʯ����Ӧ�ķ���ʽΪMg2++ NH4++ PO43-= MgNH4 PO4���÷�������Ҫ������ˮ��pHΪ7.5-10����pH����10.7�����ʯ�IJ������͡���ԭ������� �� ���뷽��һ��ȣ����������ŵ��� �� ��
��3��������ϩ��ӡˢ����֯����ҵӦ�ù㷺��Ϊ�˼�����Ի�����Ӱ�죬�ɽ�������ϩ�ڶ������ѱ�Ĥ�ϴ����⣬�䷴Ӧ�Ļ������£�
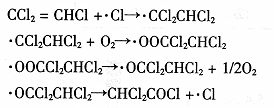
�÷�Ӧ���ܻ�ѧ����ʽΪ �� ��
��1��Ⱦ�Ϲ�ҵ�ŷŵķ�ˮ�к��д����ж���NO2-�������ڼ��������¼������۳�ȥ(���ȴ�����ķ�ˮ�������ʹʪ���ɫʯ����ֽ����������)����ȥNO2-���ӵ����ӷ���ʽ��
�� ��
��2����ˮ�е�N��PԪ�������ˮ�帻Ӫ��������Ҫ���أ�ũҩ���ŷŵķ�ˮ�г����н϶��NH4+��PO43-��һ�����ͨ�����ַ��������ȥ��
�ٷ���һ����Ca(OH)2��CaOͶ�ӵ��������ķ�ˮ�У���������ƣ��Ӷ����л��ա���������ķ�ˮ��c��Ca2+��=2��10-7mol/Lʱ����Һ��c��PO43-��= ��
(��֪����ʱ����SP��Ca3��PO4��2��=2��10-33)
�ڷ��������ڷ�ˮ�м���þ��ҵ��ˮ���Ϳ������ɸ�Ʒλ����ʯ�������ʯ����Ӧ�ķ���ʽΪMg2++ NH4++ PO43-= MgNH4 PO4���÷�������Ҫ������ˮ��pHΪ7.5-10����pH����10.7�����ʯ�IJ������͡���ԭ������� �� ���뷽��һ��ȣ����������ŵ��� �� ��
��3��������ϩ��ӡˢ����֯����ҵӦ�ù㷺��Ϊ�˼�����Ի�����Ӱ�죬�ɽ�������ϩ�ڶ������ѱ�Ĥ�ϴ����⣬�䷴Ӧ�Ļ������£�
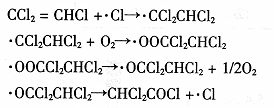
�÷�Ӧ���ܻ�ѧ����ʽΪ �� ��
��1��2Al+OH-+NO2-+2H2O=2AlO2-+NH3?H2O
��2����5��10-7
�ڵ�pH����10.7ʱ,��Һ�е�NH4+��Mg2+����OH����Ӧ
�ܳ�ֳ�ȥ��ˮ�еĵ����������þ��ҵ�ķ�ˮ��
��3��2CCl2=CHCl+O2=2CHCl2COCl
��ÿ��2�֣���10�֣�
��2����5��10-7
�ڵ�pH����10.7ʱ,��Һ�е�NH4+��Mg2+����OH����Ӧ
�ܳ�ֳ�ȥ��ˮ�еĵ����������þ��ҵ�ķ�ˮ��
��3��2CCl2=CHCl+O2=2CHCl2COCl
��ÿ��2�֣���10�֣�
��1��������Ϣ�ɵã�����ʹʪ���ɫʯ����ֽ����������ΪNH3�����Է�Ӧ�����ӷ���ʽΪ��2Al+OH-+NO2-+2H2O=2AlO2-+NH3?H2O
��2���٣�SP��Ca3��PO4��2��=c(Ca2+)3��c(PO43-)2=(2��10-7)2��c(PO43-)2�ã�c��PO43-��=5��10-7��
��pH����10.7�����ʯ�IJ������ͣ���ԭ������ǣ���Һ�е�NH4+��Mg2+����OH����Ӧ
�뷽��һ��ȣ����������ŵ��ǣ��ܳ�ֳ�ȥ��ˮ�еĵ����������þ��ҵ�ķ�ˮ��
��3�����ݷ�Ӧ������������ʽ���ӵø÷�Ӧ���ܻ�ѧ����ʽΪ��2CCl2=CHCl+O2=2CHCl2COCl
��2���٣�SP��Ca3��PO4��2��=c(Ca2+)3��c(PO43-)2=(2��10-7)2��c(PO43-)2�ã�c��PO43-��=5��10-7��
��pH����10.7�����ʯ�IJ������ͣ���ԭ������ǣ���Һ�е�NH4+��Mg2+����OH����Ӧ
�뷽��һ��ȣ����������ŵ��ǣ��ܳ�ֳ�ȥ��ˮ�еĵ����������þ��ҵ�ķ�ˮ��
��3�����ݷ�Ӧ������������ʽ���ӵø÷�Ӧ���ܻ�ѧ����ʽΪ��2CCl2=CHCl+O2=2CHCl2COCl

��ϰ��ϵ�д�
�����Ŀ
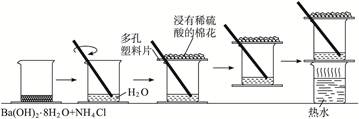
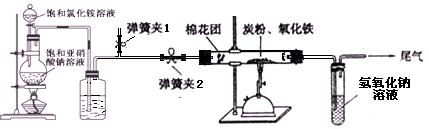
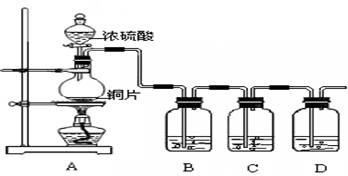
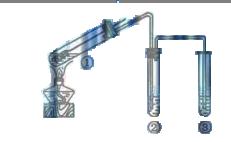
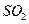 ����
���� ���ɣ�����м�ˮ���۲���ɫ
���ɣ�����м�ˮ���۲���ɫ