��Ŀ����
����Ŀ�������ݱ������ҹ����Ϻ��������������еĿ�ȼ���������ˮ����)�Բɻ�óɹ���������һ����Ҫ�Ļ���ԭ�ϡ�
��1����������������������ʵ���Ҫ��ʽ������������������¶��֣�
ˮ����������CH4(g)+H2O(g)![]() CO(g)+3H2(g) ��H1=+205.9kJ��mol-1 ��
CO(g)+3H2(g) ��H1=+205.9kJ��mol-1 ��
CO(g)+H2O(g)![]() CO2(g)+H2(g) ��H2=��41.2kJ��mol-1 ��
CO2(g)+H2(g) ��H2=��41.2kJ��mol-1 ��
������̼������CH4(g)+CO2(g)![]() 2CO(g)+2H2(g) ��H3 ��
2CO(g)+2H2(g) ��H3 ��
��Ӧ ���Է����е�������________________����H3 =_____________kJ��mol-1��
�������Ĺ̶�һֱ�ǿ�ѧ���о�����Ҫ���⣬�ϳɰ������˹��̵��Ƚϳ���ļ�������ԭ��ΪN2(g)+3H2(g)![]() 2NH3(g)
2NH3(g)
��2���ڲ�ͬ�¶ȡ�ѹǿ����ͬ���������£���ʼʱN2��H2�ֱ�Ϊ0.1mol��0.3molʱ��ƽ��������а��������������������ͼ��ʾ��
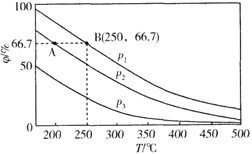
�����У�p1��p2��p3�ɴ�С��˳����________����ԭ����_________________��
�����ֱ���vA(N2)��vB(N2)��ʾ�ӷ�Ӧ��ʼ����ƽ��״̬A��Bʱ�Ļ�ѧ��Ӧ���ʣ���vA(N2)______ vB(N2)������>����<������=����
������250����p1Ϊ105Pa�����£���Ӧ�ﵽƽ��ʱ���������Ϊ1L�����������B��N2�ķ�ѹp(N2)Ϊ_________Pa (��ѹ=��ѹ�����ʵ�������������һλС��)��
���������������(S2O42��)Ϊý�飬ʹ�ü�ӵ绯ѧ��Ҳ�ɴ���ȼú�����е�NO��װ����ͼ��ʾ��
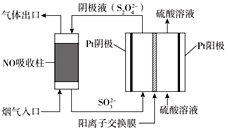
���������ĵ缫��ӦʽΪ_____________________________��
��NO����ת�������Ҫ����ΪNH4+����ͨ��ʱ��·��ת����0.3mole�������ͨ����������������յ�NO�ڱ�״���µ����Ϊ_______mL��
���𰸡� ���� +247.1 p1>p2>p3 �¶���ͬʱ������ѹǿ��ѧƽ��������Ӧ�����ƶ�����ƽ�������а����������Խ��ѹǿԽ�� < 8.3��103 2SO32-+4H++2e-=S2O42-+2H2O 1344
������������(1)��Ӧ ��CH4(g)+H2O(g)![]() CO(g)+3H2(g) ��H1=+205.9kJ��mol-1����H��0����S��0����ӦҪ�Է���������G=��H-T��S��0����Ҫ���£����Է����У����ݢ�CH4(g)+H2O(g)
CO(g)+3H2(g) ��H1=+205.9kJ��mol-1����H��0����S��0����ӦҪ�Է���������G=��H-T��S��0����Ҫ���£����Է����У����ݢ�CH4(g)+H2O(g)![]() CO(g)+3H2(g) ��H1=+205.9kJ��mol-1����CO(g)+H2O(g)
CO(g)+3H2(g) ��H1=+205.9kJ��mol-1����CO(g)+H2O(g)![]() CO2(g)+H2(g) ��H2=��41.2kJ��mol-1�����ݸ�˹���ɣ�����-�ڵã�CH4(g)+CO2(g)
CO2(g)+H2(g) ��H2=��41.2kJ��mol-1�����ݸ�˹���ɣ�����-�ڵã�CH4(g)+CO2(g)![]() 2CO(g)+2H2(g) ��H3=(+205.9kJ��mol-1)-(��41.2kJ��mol-1)=+247.1 kJ��mol-1���ʴ�Ϊ�������� +247.1��
2CO(g)+2H2(g) ��H3=(+205.9kJ��mol-1)-(��41.2kJ��mol-1)=+247.1 kJ��mol-1���ʴ�Ϊ�������� +247.1��
����(2)����N2+3H22NH3��֪������ѹǿ��ƽ�������ƶ�����ͼ���֪����ͬ�¶��£�ƽ��������а����������(��)ΪP1��P2��P3�����ѹǿ��ϵ��p1��p2��p3���ʴ�Ϊ��p1��p2��p3���¶���ͬʱ����ѹƽ�������ƶ�����ѹǿԽ��ƽ�������а����������Խ��ѹǿԽ��
���¶�Խ��ѹǿԽ��Ӧ����Խ��p1��p2����ͼ��֪��B��Ӧ���¶ȡ�ѹǿ����Ӧ���ʴʴ�Ϊ������
�� N2 + 3H2 2NH3
��ʼ(mol) 0.1 0.3 0
ת��(mol) x 3x 2x
ƽ��(mol) 0.1-x 0.3-3x 2x
![]() =0.667��x=0.08mol����B��N2�ķ�ѹp(N2)Ϊ
=0.667��x=0.08mol����B��N2�ķ�ѹp(N2)Ϊ![]() ��105Pa=8.3��103 Pa���ʴ�Ϊ��8.3��103��
��105Pa=8.3��103 Pa���ʴ�Ϊ��8.3��103��
��������ͼ��֪��������ͨ��Һ����Ҫ��SO32-��������Ҫ��S2O42-�������������缫��ӦʽΪ2SO32-+4H++2e-=S2O42-+2H2O���ʴ�Ϊ��2SO32-+4H++2e-=S2O42-+2H2O��
������NO����ת�������Ҫ����ΪNH4+��NO��NH4+��5e-������·��ת��ת��0.3mole-����Ҫ����NO0.06mol����״�������Ϊ0.06mol��22.4L/mol =1344mL���ʴ�Ϊ��1344��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ�������й�ʵ������ͽ��ͻ���۶���ȷ����
ѡ�� | ʵ����� | ���� | ���ͻ���� |
A | ����۵�����ˮ��Һ�е�����������Cu(OH)2����Һ������ | ��ש��ɫ�������� | �����Ǿ��л�ԭ�� |
B | NaAlO2��Һ��NaHCO3��Һ��� | ��ɫ��״�������� | ����ˮ����ٽ����������������� |
C | ������NO2���ܱղ������������ˮ�� | ����ɫ���� | ��Ӧ2NO2 ����H<0 |
D | ��ʢ��1mL0.l mol/L��AgNO3��Һ�м���10��0.1mol/L��NaCl��Һ�����ټ���10��0.1mol/L��NaI��Һ������ | �����ɰ�ɫ�������������ɫ���� | Ksp(AgI)sp(AgCl) |
A. A B. B C. C D. D

