��Ŀ����
A��B��C��D�����ֳ������ʣ�B��DΪ����������A��CΪ���壬���ӦԪ�ص�ԭ�������������ס��ҡ���Ϊ�������������֮������ͼ��ʾת����ϵ��

�ش��������⣺
��1��A�ĵ���ʽ ��
��2��D�����Һ��Ӧ�Ļ�ѧ����ʽ ��
��3������ˮ��Һ�� �ԣ�������������CO2��Ӧ���ӷ���ʽ ��
��4��һ�������£�2mol B��3mol D��������Wǡ�÷�Ӧ����W�Ļ�ѧʽ ��

�ش��������⣺
��1��A�ĵ���ʽ
��2��D�����Һ��Ӧ�Ļ�ѧ����ʽ
��3������ˮ��Һ��
��4��һ�������£�2mol B��3mol D��������Wǡ�÷�Ӧ����W�Ļ�ѧʽ
���㣺������ƶ�
ר�⣺�ƶ���
������A��B��C��D�����ֳ������ʣ����ӦԪ�ص�ԭ�������������ס��ҡ���Ϊ���������B��DΪ����������A��CΪ���壬B����NaOH��Һ��Ӧ����BӦΪAl��AΪH2����ΪNaAlO2��D�ڸ�������ˮ������Ӧ��DӦΪFe������ΪFe3O4��D��C��ȼ�����ɼף�CΪCl2����ΪFeCl3���Դ˽����⣮
���
�⣺A��B��C��D�����ֳ������ʣ����ӦԪ�ص�ԭ�������������ס��ҡ���Ϊ���������B��DΪ����������A��CΪ���壬B����NaOH��Һ��Ӧ����BӦΪAl��AΪH2����ΪNaAlO2��D�ڸ�������ˮ������Ӧ��DӦΪFe������ΪFe3O4��D��C��ȼ�����ɼף�CΪCl2����ΪFeCl3��
��1�������Ϸ�����֪AΪH2�������ʽΪ��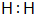 ���ʴ�Ϊ��
���ʴ�Ϊ��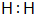 ��
��
��2��D�����Һ��Ӧ�Ļ�ѧ����ʽΪ��Fe+2FeCl3=3FeCl2���ʴ�Ϊ��Fe+2FeCl3=3FeCl2��
��3����ΪNaAlO2��Ϊǿ�������Σ�����Һ�з���AlO2-+2H2O?Al��OH��3+OH-����Һ�ʼ��ԣ�NaAlO2��Һ�����CO2��Ӧ���ӷ���ʽΪ��AlO2-+CO2+2H2O=Al��OH��3��+HCO3-��
�ʴ�Ϊ���AlO2-+CO2+2H2O=Al��OH��3��+HCO3-��
��4��һ�������£�2molAl��3molFe��һ��������Wǡ�÷�Ӧ����W��Fe�Ļ��ϼ�Ϊx������ת�Ƶ�����Ŀ��ȿ�֪2��3=3��x-0����x=2����WΪFeO��
�ʴ�Ϊ��FeO��
��1�������Ϸ�����֪AΪH2�������ʽΪ��
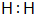 ���ʴ�Ϊ��
���ʴ�Ϊ��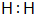 ��
����2��D�����Һ��Ӧ�Ļ�ѧ����ʽΪ��Fe+2FeCl3=3FeCl2���ʴ�Ϊ��Fe+2FeCl3=3FeCl2��
��3����ΪNaAlO2��Ϊǿ�������Σ�����Һ�з���AlO2-+2H2O?Al��OH��3+OH-����Һ�ʼ��ԣ�NaAlO2��Һ�����CO2��Ӧ���ӷ���ʽΪ��AlO2-+CO2+2H2O=Al��OH��3��+HCO3-��
�ʴ�Ϊ���AlO2-+CO2+2H2O=Al��OH��3��+HCO3-��
��4��һ�������£�2molAl��3molFe��һ��������Wǡ�÷�Ӧ����W��Fe�Ļ��ϼ�Ϊx������ת�Ƶ�����Ŀ��ȿ�֪2��3=3��x-0����x=2����WΪFeO��
�ʴ�Ϊ��FeO��
���������⿼��������ƶϣ��Ѷ��еȣ���Ӧ������ת����ϵΪ�������ͻ�ƿڣ�����������ʵ����ʣ�ѧϰ��ע���ȹػ���֪ʶ�Ļ��ۣ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
��NAΪ�����ӵ�����������˵����ȷ���ǣ�������
| A��23g������������ȫȼ��ʧȥ������Ϊ0.5NA |
| B��2mol?L-1��AlCl3��Һ�к�Al3+��Ϊ2NA |
| C��78g��������������������̼��Ӧ��ת�Ƶ�����ΪNA�� |
| D�����³�ѹ�£�22.4L��������NA����ԭ�� |
�������ʼ���ķ���������ͽ��ۣ����д�����ǣ�������
| A��ij����Һ�м�����ˮ���ټ���KSCN��Һ����Һ����Ѫ��ɫ����ԭ��Һ�к���Fe2+ |
| B��ij�μ���Ũ������������Һ�����ȣ��ܲ���ʹ��ʪ�ĺ�ɫʯ����ֽ���������壬���������� |
| C������������غ�Ũ���ᷴӦ��������������ʪ��ĵ��۵⻯����ֽ�����������������Cl2 |
| D���ýྻ�IJ�˿պȡ����Һ�����ڻ��������գ��ܹ۲쵽����ʻ�ɫ������Һ��һ�����������ӣ����ܺ��м����� |
�����İ뾶�Ӵ�С��˳�����е��ǣ�������
| A��Mg��Al��Na |
| B��Li��Na��K |
| C��Cl��S��P |
| D��N��O��F |
��������������Һ�е�ת����ϵ��ͼ��ʾ�� �����ײ������ǣ�������
�����ײ������ǣ�������
 �����ײ������ǣ�������
�����ײ������ǣ�������| A��CO2 |
| B��NH4+ |
| C��Al3+ |
| D��SiO2 |
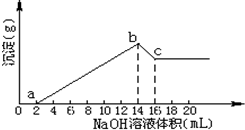 ��һ��������Mg��Al�Ͻ�ȫ���ܽ���500mL�����У�����仯���ƣ���ȡ10mL��Ӧ�����Һ����1mol/LNaOH��Һ�ζ�����ͼ��ϵ��
��һ��������Mg��Al�Ͻ�ȫ���ܽ���500mL�����У�����仯���ƣ���ȡ10mL��Ӧ�����Һ����1mol/LNaOH��Һ�ζ�����ͼ��ϵ�� ����к͵ζ�������ʵ�������Ź㷺��Ӧ�ã�����ʱ����0.250mol?L-1 NaOH��Һ�ζ�25.0mL��һԪ��HR��Һʱ����Һ��pH�仯�����ͼ��ʾ������a���ʾ��������ǡ����ȫ��Ӧ����ش��������⣺
����к͵ζ�������ʵ�������Ź㷺��Ӧ�ã�����ʱ����0.250mol?L-1 NaOH��Һ�ζ�25.0mL��һԪ��HR��Һʱ����Һ��pH�仯�����ͼ��ʾ������a���ʾ��������ǡ����ȫ��Ӧ����ش��������⣺