��Ŀ����
4�������й����ʼ����ʵ�������ȷ���ǣ�������| ѡ�� | ʵ �� �� �� �� �� �� | ʵ �� �� �� |
| A | ��ij��Һ�м��������ữ���Ȼ�����Һ���а�ɫ�������� | ����Һ��һ������SO${\;}_{4}^{2-}$ |
| B | ��ij��Һ�м������ᣬ�ܲ���ʹ����ʯ��ˮ����ǵ����� | ����Һ��һ����CO32- |
| C | ��ij��Һ�м�������NaOHϡ��Һ����ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ���ֽ����ɫ | ����Һ��һ������NH4+ |
| D | �ø���ྻ�IJ�˿պȡij��Һ���ھƾ��ƻ��������գ�����ɫ�ܲ����۲쵽�������ɫ | ����Һһ�����м�Ԫ�أ����ܺ�����Ԫ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
���� A����ɫ��������ΪAgCl��
B����Һ�п��ܴ���HCO3-��CO32-��
C�������NH4+��Ӧ����Ũ����������Һ���Ҽ��ȣ�
D������ɫ�ܲ����۲쵽�������ɫ��˵�����м����ӣ���ɫ��������ȥ�ƹ⣮
��� �⣺A����ɫ��������ΪAgCl����Ӧ�ȼ������������ټ��Ȼ���������������ӣ�ʵ��Ľ���ӦΪ��Һ�п��ܺ���SO42-���������ӣ������߲���ͬʱ���ڣ���A����
B����ij��Һ�м���ϡ���ᣬ������ʹ����ʯ��ˮ����ǵ���ɫ��ζ���壬����Ϊ������̼������Һ�п��ܴ���HCO3-��CO32-����B����
C��������������ˮ�������NH4+��Ӧ����Ũ����������Һ���Ҽ��ȣ���C����
D������ɫ�ܲ����۲쵽�������ɫ��˵�����м����ӣ���ɫ��������ȥ�ƹ⣬��D��ȷ��
��ѡD��
���� ���⿼�黯ѧʵ�鷽�������ۣ�Ϊ��Ƶ���㣬������ѧ���ķ���������ʵ�������Ŀ��飬ע����ճ������ӵļ��鷽��������ʱע���ų��������ӵĸ��ţ��ѶȲ���

��ϰ��ϵ�д�
 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�
�����Ŀ
12�����й����������������ȷ���ǣ�������
| A�� | ����������һ���Ǽ���������ǽ���������һ�������������� | |
| B�� | ����������һ���ǽ��������� | |
| C�� | ���������ﶼ����ˮ�������ɼ� | |
| D�� | ����������һ���Ƿǽ��������� |
13�����ݱ���Ϊ���й���֮��������������ɫ��������ݡ���2015��5�³ɹ��ٰ��˵�������
�������ϣ����ʿ��ﱦʯ�����ᣮ����չʾ��ɫ���ͷס���״����Ľ��������
�н��ұ�������Ȼ�ԭ�����ǣ�������
�������ϣ����ʿ��ﱦʯ�����ᣮ����չʾ��ɫ���ͷס���״����Ľ��������
�н��ұ�������Ȼ�ԭ�����ǣ�������
| A�� | 2NaCl�����ڣ�=2Na+Cl2�� | B�� | WO3+3H2 $\frac{\underline{\;��\;}}{\;}$ W+3H2O | ||
| C�� | Fe+CuSO4=Cu+FeSO4 | D�� | 2Ag2O $\frac{\underline{\;��\;}}{\;}$4Ag+O2�� |
10��400��ʱ����һ�ܱ�������ͨ�� 4molSO2�� 2molO2����Ӧ�ﵽƽ��ʱ��������ѹǿ�Ƿ�Ӧǰ���ķ�֮������ʱ SO2��ת����Ϊ��������
| A�� | 75% | B�� | 25% | C�� | 37.5% | D�� | 81% |
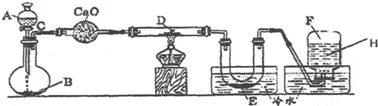
 ʵ��������500mL 0.2mol��L-1Na2S04��Һ��ʵ������������£�
ʵ��������500mL 0.2mol��L-1Na2S04��Һ��ʵ������������£�