��Ŀ����
��15�֣���ѧ������̽���������صĹ����У���ʶ������ϸ������ɺ�Ԫ���������������е���ϵ��Լռ����������99.97%��11�ִ���Ԫ��ȫ��λ��Ԫ�����ڱ�ǰ20��Ԫ�أ�����0.03%����10�������岻��ȱ�ٵ���Ԫ����ɡ�����a~g 7�ֶ�����Ԫ�أ��dz������ء��������������Ԫ�أ�������Ԫ�����ڱ��е�λ�����£���ݴ˻ش��������⣺
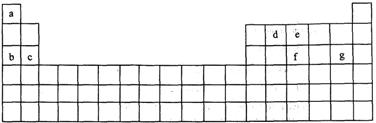
��1��Ԫ�ص�ԭ�Ӽ䷴Ӧ�������γ����Ӽ����������еģ� ����Ԫ�ص�ԭ�Ӽ䷴Ӧ�����γɹ��ۼ����������еģ� ��
A��c��f B��b��g C��d��g D��d��e
��2��������a~g�γɵĸ������У�����ԭ�Ӷ����������Ϊ8���ӽṹ����A��ea3 B��ag C��fg3 D��dg4
��3����11��Ԫ�صļ��ַǽ���Ԫ�ع��ɵ����ӻ�����ĵ���ʽΪ___ ___��
��4��c��e���γ�һ�ֻ�����Ը���ԭ�ӽṹд���û�����Ļ�ѧʽ����������������
��ѧ������Ϊ������������ϡ���ᷴӦ�ķ���ʽΪ ���������������� ��

��1��Ԫ�ص�ԭ�Ӽ䷴Ӧ�������γ����Ӽ����������еģ� ����Ԫ�ص�ԭ�Ӽ䷴Ӧ�����γɹ��ۼ����������еģ� ��
A��c��f B��b��g C��d��g D��d��e
|
��3����11��Ԫ�صļ��ַǽ���Ԫ�ع��ɵ����ӻ�����ĵ���ʽΪ___ ___��
��4��c��e���γ�һ�ֻ�����Ը���ԭ�ӽṹд���û�����Ļ�ѧʽ����������������
��ѧ������Ϊ������������ϡ���ᷴӦ�ķ���ʽΪ ���������������� ��
(��15��)����1��B ��2�֣���C ��2�֣�������2��CD��2�֣�
��3��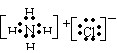 ��2�֣�
��2�֣�
��4��Mg3N2��2�֣��������Ӽ� ��2�֣�
Mg3N2+8HCl=3MgCl2+2NH4Cl��3�֣�
��3��
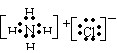 ��2�֣�
��2�֣���4��Mg3N2��2�֣��������Ӽ� ��2�֣�
Mg3N2+8HCl=3MgCl2+2NH4Cl��3�֣�
��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 ʵ�飬����������ļ�ơ���֪������BGO��,�ദ�������̬������BGO��,��ļ�̬��������γ�ij�ֹ����Ȼ���ʱ���ʵļ�̬��ͬ���ڴ��Ȼ���������������8�������ȶ��ṹ����BGO�ɿ����������������Ԫ�ص����������γɵĸ������������BGO����Ļ�ѧʽ��,����������������������������ͬ��
ʵ�飬����������ļ�ơ���֪������BGO��,�ദ�������̬������BGO��,��ļ�̬��������γ�ij�ֹ����Ȼ���ʱ���ʵļ�̬��ͬ���ڴ��Ȼ���������������8�������ȶ��ṹ����BGO�ɿ����������������Ԫ�ص����������γɵĸ������������BGO����Ļ�ѧʽ��,����������������������������ͬ��
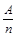 ��KΪA��n�ı�ֵ��������������ȷ����
��KΪA��n�ı�ֵ��������������ȷ����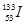 �������й�������ȷ���� �� ��
�������й�������ȷ���� �� �� Ԫ��A��B��C��ԭ���������ε��������ǵ�ԭ������������֮��Ϊ10��A��Cͬ���壬Bԭ�ӵ���������������Aԭ�ӵĴ�����������������������ȷ����
Ԫ��A��B��C��ԭ���������ε��������ǵ�ԭ������������֮��Ϊ10��A��Cͬ���壬Bԭ�ӵ���������������Aԭ�ӵĴ�����������������������ȷ����