��Ŀ����
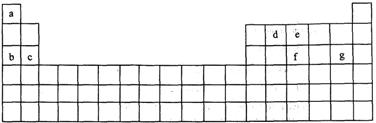
(16�֣�����ΪԪ�����ڱ��е�һ���֣���ע�����������û�ѧ����ش��������⣺
��8��Ԫ���У���ѧ��������õ��� ���ǽ�������ǿ���� ��
�Ƣ٢ڢݵ�����������Ӧˮ����ļ�����ǿ������˳��Ϊ ��
�Ǣڢۢ����γɵļ����Ӱ뾶�ɴ�С��˳��Ϊ ��
�Ȣ��⻯�ﳣ������Ԫ�آڵĹ������ﷴӦ�����ӷ���ʽΪ ��
�ɢٺ͢��γɵĵ���ɫ����ĵ���ʽΪ ��������ѧ��������Ϊ������Ӽ������ۼ����� ��
�ʢٺ͢ݵ�����������Ӧˮ�������Ӧ�����ӷ���ʽΪ ��
 | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | | | | | | �� | �� | |
| 3 | �� | �� | �� | | | | | �� |
| 4 | �� | �� | | | | | | |
�Ƣ٢ڢݵ�����������Ӧˮ����ļ�����ǿ������˳��Ϊ ��
�Ǣڢۢ����γɵļ����Ӱ뾶�ɴ�С��˳��Ϊ ��
�Ȣ��⻯�ﳣ������Ԫ�آڵĹ������ﷴӦ�����ӷ���ʽΪ ��
�ɢٺ͢��γɵĵ���ɫ����ĵ���ʽΪ ��������ѧ��������Ϊ������Ӽ������ۼ����� ��
�ʢٺ͢ݵ�����������Ӧˮ�������Ӧ�����ӷ���ʽΪ ��
(16�֣���Ar��F����KOH��NaOH��Al��OH)3����K+��Ca2+��Mg2+��
��2H2O+2Na2O2=4Na+ +4OH- +O2������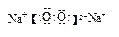 �����Ӽ����ۼ���
�����Ӽ����ۼ���
��OH- +Al��OH)3 =AlO2- +2H2O
|
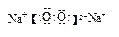 �����Ӽ����ۼ���
�����Ӽ����ۼ�����OH- +Al��OH)3 =AlO2- +2H2O
��

��ϰ��ϵ�д�
�����Ŀ
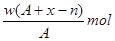
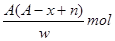
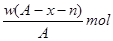
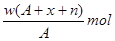
 ����2����
����2���� �Իش�
�Իش� W_________��
W_________��