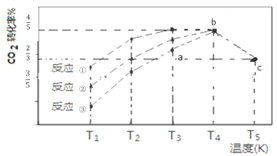
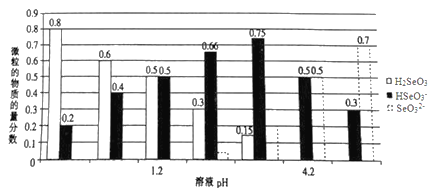
��Ŀ����
����Ŀ����֪���ᡢ��ˮ���ܶ�������ˮ���Ĺ�ϵ��ͼ��ʾ�����������백ˮ��һ�ݣ�����ݱ�����Ϣ���ش��������⣺
���ʵ����ʵ���Ũ��/molL-1 | ���ʵ��������� | ��Һ���ܶ�/gcm-3 | |
���� | c1 | w1 | ��1 |
��ˮ | c2 | w2 | ��2 |

��1�������������������w1Ϊ_____(��д��λ���ú�c1����1�Ĵ���ʽ��ʾ)��
��2�����ʵ���Ũ��Ϊc1mol��L-1��������Ϊw1��������ˮ��������(��Ϻ���Һ����仯���Բ���)��������Һ�����ʵ���Ũ��Ϊ_______mol��L-1����������_______w1/2(����ڡ�����С�ڡ����ڡ�����ͬ)��
��3����������Ϊw2�İ�ˮ��w2/5�İ�ˮ��������ϣ�������Һ���ܶ�_____��2 gcm-3��
��4�������700����İ����ܽ���1���ˮ���γɰ�ˮ������Һ����Һ���ܶ�Ϊd g/cm3�������Һ�����ʵ���Ũ��Ϊ________���ú���d�ı���ʽ��ʾ����
���𰸡�(9.8c1/��1)%��98c1/1000��1 c1/2 ���� ���� 1000d/49 mol��L��1
��������
��1������c��1000�Ѧ�/M���й�ʽ���μ��㣻
��2������ϡ�Ͷ��ɣ�ϡ��ǰ��������������ʵ������������䣬�ݴ˼���ϡ�ͺ���Һ��Ũ�Ⱥ�����������
��3����������Ϊw2�İ�ˮ��w2/5�İ�ˮ��������Ϻ���Һ��Ũ��С��w2����ͼ��֪����ˮ��Ũ��Խ���ܶ�ԽС���ݴ��жϻ�Ϻ���Һ���ܶ�����2 gcm-3��ϵ��
��4������n��V/Vm��c��n/V�����Һ���������ܶȼ��㰱ˮ��Ũ�ȡ�
��1������c��1000�Ѧ�/M��֪���������������w1��98c1/1000��1��
��2����������ˮ�����ΪVL�����Ϻ���Һ�������Ϊ2VL������ϡ�Ͷ��ɣ�ϡ��ǰ��������������ʵ������䣬ϡ�ͺ�������Һ��Ũ��Ϊ��VL��c1mol��L��1/2VL��0.5c1mol/L����������Ϊw1��������ˮ�������ϣ�ˮ������С��������Һ����������������С��ԭ������2�������������������w1/2��
��3����������Ϊw2�İ�ˮ��w2/5�İ�ˮ��������ϣ���Ϻ���Һ����������С��w2����ͼ��֪����ˮ��Ũ��Խ���ܶ�ԽС���ʻ�Ϻ���Һ���ܶȴ�����2 gcm-3��
��4��������700L�İ����ܽ���1Lˮ���γɰ�ˮ������Һ�����ʵ����ʵ�����![]() ��������
��������![]() ����Һ��������1000g+
����Һ��������1000g+![]() ����Һ���ܶ�Ϊd g/cm3�������Һ�������
����Һ���ܶ�Ϊd g/cm3�������Һ�������![]() ��������ʵ���Ũ��Ϊ
��������ʵ���Ũ��Ϊ ��
��

 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д� â���̸������Ծ�ϵ�д�
â���̸������Ծ�ϵ�д�����Ŀ������ʵ��������ʵ����ۻ�ԭ����һ�µ���
ѡ�� | ʵ������ | ʵ����ۻ�ԭ�� |
A | ����������ĭ�������� | 3HCO3-+Al3+===Al(OH)3��+3CO2�� |
B | ��AgCl����Һ�е�������KI��Һ���л�ɫ�������� | ˵��KSP��AgCl����KSP��AgI�� |
C | ��NaHS��Һ�е����̪����Һ���ɫ | HS-ˮ��̶ȴ��ڵ���̶� |
D | Na2CO3��Һ�еμӷ�̪�ʺ�ɫ | CO32-+2H2O |
A. A B. B C. C D. D
����Ŀ��ijС����Ʋ�ͬʵ�鷽���Ƚ�Cu2+��Ag+ �������ԡ�
��1������1��ͨ���û���Ӧ�Ƚ�
���ữ��AgNO3��Һ����ͭ˿��������ɫ���壬��Һ��������Ӧ�����ӷ���ʽ��_______��˵��������Ag+��Cu2+��
��2������2��ͨ��Cu2+��Ag+ �ֱ���ͬһ���ʷ�Ӧ���бȽ�
ʵ�� | �Լ� | ��ż����� | |
�Թ� | �ι� | ||
| 1.0 mol/L KI��Һ | 1.0 mol/L AgNO3��Һ | ������ɫ��������Һ��ɫ |
1.0 mol/L CuSO4��Һ | ������ɫ����A����Һ��� | ||
�� �����飬������Һ����I2����ɫ������________��
�� �����飬������Һ��I2���Ʋ�Cu2+������������ɫ����A��CuI��ȷ��A��ʵ�����£�
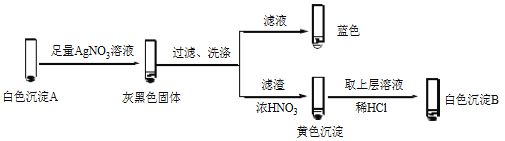
a��������Һ��I2����Һ����ɫ˵����Һ����________�������ӷ��ţ���
b����ɫ����B��________��
c����ɫ����A��AgNO3��Һ��Ӧ�����ӷ���ʽ��____��˵��������Ag+��Cu2+��
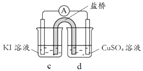
��3����������2��Ag+ δ������I- ����Cu2+������I-��ԭ�����ʵ�����£�
��� | ʵ��1 | ʵ��2 | ʵ��3 |
ʵ�� |
|
|
|
���� | �����Ա仯 | a����Һ�Ͽ���ػ�ɫ,b�е缫 ����������������ָ��ƫת | c����Һ������dz��ɫ�� ������ָ��ƫת |
���缫��Ϊʯī����ҺŨ�Ⱦ�Ϊ 1 mol/L��b��d����ҺpH��4��
�� a����Һ���ػ�ɫ��ԭ����_______���õ缫��Ӧʽ��ʾ����
�� ��ʵ��3������˵��Cu2+������I-�������ǿ����е�����Ҳ���������ã����ʵ��֤ʵ�˸����ݣ�ʵ�鷽����������_______��
�� ����2�У�Cu2+������I-,��Ag+δ������I-��ԭ��_______��
�����ϣ�Ag+ + I- = AgI�� K1 =1.2��1016��2Ag+ + 2I- = 2Ag��+ I2 K2 = 8.7��108��