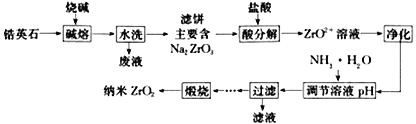
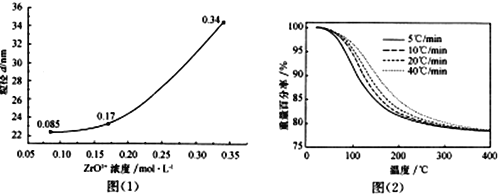
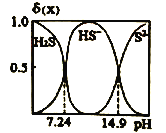
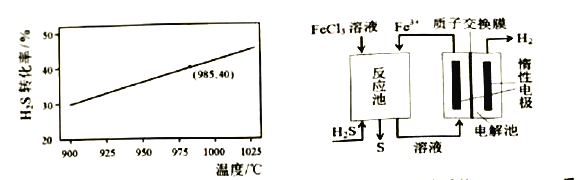
��Ŀ����
����Ŀ�����ݵ���ƽ�ⳣ��(��Ka��ʾ)�Ĵ�С�����жϵ���ʵ����ǿ����25��ʱ���й����ʵĵ���ƽ�ⳣ�����±���ʾ��
��ѧʽ | HF | H2CO3 | HClO | H2SO3 |
����ƽ�ⳣ��(Ka) | Ka=7.2��10-4 | Ka1=4.2��10-7 Ka2=5.6��10-11 | Ka=3.0��10-8 | Ka1=1.0��10-2 Ka2=5.0��10-8 |
��1��25��ʱ��ͬŨ�ȵ�HF��H2CO3��HClO������Һ�У�pH������____________(д��ѧʽ)��
��2����0.1mol��L-1��HF��Һ1mL��ˮϡ����10mL(�����¶Ȳ���)�����и����������___________(��д��ĸ)��
A��c(H+) B��c(H+)��c(OH-) C��c(H+)/c(HF) D��c(OH-)/c(H+)
��3��25��ʱ���������ʵ���Ũ�Ⱦ�Ϊ0.1mol��L-1������������Һ��
��Na2CO3��Һ ��NaHCO3��Һ ��NaF��Һ ��NaClO��Һ��
���������ж���pH�ɴ�С��˳����_________________________(��д���)��
��4������H2SO3�ĵ���ƽ�ⳣ��,����25��ʱ,0.05mol��L-1Na2SO3��Һ��pH=__________��
��ijNa2SO3��NaHSO3�Ļ��Һ�����ԣ�����Һ��c(SO32-)__________c(HSO3-)(����ڡ�����С�ڡ�����")��
���𰸡� HClO C D ���ܣ��ڣ��� 10 С��
����������1���鿴������ĵ���ƽ�ⳣ������Ԫ���ῴ��һ�����룻ƽ�ⳣ��Խ������Խǿ��pHԽС����ȷѡ��HClO��
��2��HFΪ���ᣬ���ڵ���ƽ�⣬��ˮϡ�ͣ�ƽ�����ƣ�c(H+)��c(HF)��c(F-)���������¶Ȳ�����c(H+)��c(OH-)Ϊ����������c(OH-)������A��c(H+) ������B��c(H+)��c(OH-)������C��c(H+)/c(HF)������D��c(OH-)/c(H+)��������ȷѡ�CD��
��3�������γɸ����ε����������ǿ�������жϣ���Խ�����γɵ�����Խ��ˮ�⣬��Һ�ļ��Ծ�Խǿ����������������ĵ���ƽ�ⳣ����K(HF)> K(H2CO3) > K(HClO) > K(HCO-3)
�����ε�pH�ɴ�С��˳���ǣ�Na2CO3��Һ> NaClO��Һ> NaHCO3��Һ> NaF��Һ����ȷ��Ϊ�� ����������������
��4��ˮ�ⷴӦΪSO32-+H20![]() HSO3-+OH-����ˮ�������c(OH-)=c(HSO3-)= xmol/L ��ˮ��ƽ�ⳣ��ΪKh=c(OH-)��c(HSO3-)/c(SO32-)=KW/Ka2=1��10-14/5.0��10-8=2��10-7; X2/(0.05-x)= 2��10-7�����Ƽ�����X=10-4mol/L��c(H+)=10-10�� pH=10����Ϊ10��
HSO3-+OH-����ˮ�������c(OH-)=c(HSO3-)= xmol/L ��ˮ��ƽ�ⳣ��ΪKh=c(OH-)��c(HSO3-)/c(SO32-)=KW/Ka2=1��10-14/5.0��10-8=2��10-7; X2/(0.05-x)= 2��10-7�����Ƽ�����X=10-4mol/L��c(H+)=10-10�� pH=10����Ϊ10��
��5��HSO3-���Ӽ���ˮ�����ܵ��룬NaHSO3��Һ������ͬʱ˵��HSO3-�ĵ���̶ȴ���ˮ��̶ȣ�Na2SO3ˮ���Լ��ԣ���ˮ��������ǿ��ֻ��HSO3-����Ũ�ȴ���SO32-����Ũ�ȣ��Ż�ʹ���ߵĻ����Һ�������ԣ���Ϊ��С����

 ���ĺ����Ͼ�������ϵ�д�
���ĺ����Ͼ�������ϵ�д�����Ŀ��̽��Na2S2O3+H2SO4=Na2SO4+SO2��+S��+H2O ��Ӧ������Ӱ�����أ����������ʵ�飬����˵����ȷ����
��ƿ | 0.1molLNa2S2O3 ��Һ/mL | ����ˮ | 0.2mol/L H2SO4 ��Һ | ��Ӧ�¶� | ���dz���ʱ��/s | ��ע |
1 | 10 | 0 | 10 | 20�� | 10 | |
2 | 10 | 5 | 5 | 20�� | 16 | |
3 | 10 | 0 | 10 | 50�� | 5 | ��10 �뿪ʼ���Dz������� |
4 | 10 | 6 | 4 | 50�� | 8 |
A. �÷�ӦҲ��ͨ����SO2������仯����ʾ��ѧ��Ӧ���ʵĿ���
B. 3��ƿ��Na2S2O3 ����ʾ����Ϊ0��0lmol/(Ls)
C. ��2��ƿ��3��ƿʵ�����ɵ��¶�Խ�߷�Ӧ����Խ��
D. ��1��ƿ��4��ƿʵ�����ɵ��¶�Խ�߷�Ӧ����Խ��