��Ŀ����
���ڣ�2C4H10(g)+13O2(g)=8CO2(g)+10H2O(l)����H =��5800kJ/mol�������������
| A���÷�Ӧ�ķ�Ӧ��Ϊ��H=��5800kJ/mol���Ƿ��ȷ�Ӧ |
| B���÷�Ӧ�ġ�H������ʵ�״̬�йأ��뻯ѧ������Ҳ�й� |
| C����ʽ�ĺ���Ϊ��25�桢101kPa�£�2mol C4H10������ȫȼ������CO2��Һ̬ˮʱ�ų�����5800kJ |
| D���÷�ӦΪ����ȼ�յ��Ȼ�ѧ����ʽ���ɴ˿�֪�����ȼ����Ϊ5800kJ/mol |
D
�������������ȼ������ָ��ȫȼ��1mol��ȼ���ʱ�����ų����������ɴ˿�֪�����ȼ����Ϊ2900kJ/mol����ˣ�D����
���㣺���鷴Ӧ�����⡣

��ϰ��ϵ�д�
 �������ͬ������ϵ�д�
�������ͬ������ϵ�д�
�����Ŀ
�����뻯ѧ��Ӧ�����仯��ص���������ȷ����
| A����֪CH4(g)��2O2(g)=CO2(g)��2H2O(g) ��H��-802 kJ/mol�������ȼ����Ϊ802 kJ/mol |
| B������H2��O2����ȫȼ�գ�����H2O(g)������H2O(l)�ų��������� |
| C��ͬ��ͬѹ�£�H2(g)��Cl2(g)=2HCl(g)�ڹ��պ͵�ȼ�����µĦ�H��ͬ |
| D����ʯī�Ƚ��ʯ�ȶ���֪��C(���ʯ, s)=C(ʯī, s) ��H<0 |
�����Ȼ�ѧ����ʽ��ȷ���ǣ�ע��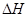 �ľ���ֵ����ȷ��( )
�ľ���ֵ����ȷ��( )
| A��C2H5OH(l)+3O2(g)=2CO2(g) +3H2O(g)����H=" ��1367.0" kJ/mol��ȼ���ȣ� |
| B��NaOH(aq) + HCl(aq)="NaCl(aq)" + H2O(l)����H= ��57.3kJ/mol���к��ȣ� |
| C��S(s) + O2(g) = SO2(g)����H= ��269.8kJ/mol����Ӧ�ȣ� |
| D��2HCl(g)=Cl2(g) + H2(g)����H=" ��" 184.6kJ/mol����Ӧ�ȣ� |
21�����������ɡ���ʯ��Դʱ����������Դʱ�������ɣ����в���������Դ���ǣ� ��
| A������ | B������ | C��̫���� | D������ |
���б仯���̣����ڷ��ȷ�Ӧ���ǣ� ��
��Һ̬ˮ���ˮ���� ������кͷ�Ӧ ��ŨH2SO4ϡ��
�ܹ���NaOH����ˮ ��H2��Cl2��ȼ�� ���������
| A���ڢۢܢ� | B���ڢۢ� | C���ڢ� | D���٢ۢ� |
��֪���ȼ��a g��Ȳ(C2H2)����ʱ����1 mol CO2�����Һ̬ˮ�����ų�����b kJ������Ȳȼ�յ��Ȼ�ѧ����ʽ��ȷ����
| A��2C2H2(g)��5O2(g)=4CO2(g)��2H2O(l)��H����4b kJ��mol-1 |
B��C2H2(g)�� O2(g)=2CO2(g)��H2O(l)��H��2b kJ��mol-1 O2(g)=2CO2(g)��H2O(l)��H��2b kJ��mol-1 |
| C��2C2H2(g)��5O2(g)=4CO2(g)��2H2O(l)��H����2b kJ��mol-1 |
| D��2C2H2(g)��5O2(g)=4CO2(g)��2H2O(l)��H����b kJ��mol-1 |
1������ȼ������Һ̬ˮ�ų�142.9 kJ��������ʾ�÷�Ӧ���Ȼ�ѧ����ʽ��ȷ����( )
| A��2H2(g)+O2(g)=2H2O(l)����H="-285.8" kJ/mol |
| B��H2(g)+1/2O2(g)=H2O(g)����H=-142.9kJ/mol |
| C��H2O(l)=H2(g)+1/2O2(g)�� ��H="+285.8" kJ/mol |
| D��2H2+O2=2H2O����H="-571.6" kJ/mol |
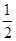 O2(g)=CO(g)����H2
O2(g)=CO(g)����H2