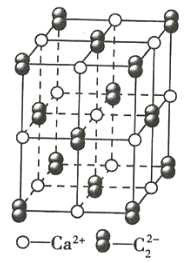
��Ŀ����
����Ŀ��A��B��C��D��E��Ԫ�����ڱ�ǰ�������еij���Ԫ�أ�ԭ�������������������Ϣ���£�(NAΪ�����ӵ�������ֵ)�����û�ѧ����ش��������⣺
Ԫ�� | �����Ϣ |
A | ��̬ԭ�ӵļ۵����Ų�ʽΪnSnnPn |
B | Ԫ��ԭ�ӵĺ���p��������s��������1�� |
C | �����������ǵ��Ӳ�����3�� |
D | �������ǵ�������Ԫ�������Ӱ뾶��С�� |
E | �۵��Ӳ��е�δ�ɶԵ�����Ϊ4 |
(1)д��DԪ�������ڱ���λ��______����̬E2+�۵��ӵ��Ų�ͼΪ_______��BԪ��������ߵĵ���������_______�Ρ�
(2)A��C�γɵ���ۻ��������ԭ�ӹ���ӻ�����Ϊ___________��
(3)A��B��C���ֻ�̬ԭ�ӵĵ�һ�������ɴ�С��˳��Ϊ_____________��B��C��D�����ӵİ뾶�ɴ�С��˳��Ϊ_________��
(4)д��C�ĺ�����18�����ӵ��⻯��ĵ���ʽ________��
(5)E������ijЩ��Ӧ�Ĵ�����CO����Eʧȥ�����ԣ�E��5CO = E(CO)5��E(CO)5�۵�Ϊ-20�棬�е�Ϊ103�棬���������ѣ��侧������Ϊ___________��
(6)��֪�е�:B2H4>A2H6 ����Ҫԭ��Ϊ____________________��
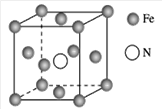
(7)���Ķ��ֻ������Ϊ���Բ��ϣ�������������һ�֣�ij�������ľ����ṹ��ͼ��ʾ�������Ļ�ѧʽΪ________���辧���߳�Ϊa cm���þ�����ܶ�Ϊ________ g��cm-3(�ú�a��NA��ʽ�ӱ�ʾ)��
���𰸡��������ڣ���A�� 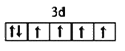 ������ sp N��O��C r(N3-)��r(O2-)��r(Al3+)
������ sp N��O��C r(N3-)��r(O2-)��r(Al3+) ![]() ���Ӿ��� ǰ�߿��γɷ��Ӽ����������ֻ�з��»��� Fe4N
���Ӿ��� ǰ�߿��γɷ��Ӽ����������ֻ�з��»��� Fe4N ![]()
��������
A��B��C��D��E��Ԫ�����ڱ�ǰ�������еij���Ԫ�أ�ԭ����������������A�Ļ�̬ԭ�ӵļ۵����Ų�ʽnsnnpn��֪��n=2����AΪCԪ�أ�C�������������ǵ��Ӳ�����3������CΪOԪ�أ�BԪ��ԭ�ӵĺ���p��������s��������1���������ԭ���������������֪��BΪNԪ�أ�DΪ�������ǵ�������Ԫ�������Ӱ뾶��С��Ԫ�أ���DΪAlԪ�أ�E�ļ۵��Ӳ��е�δ�ɶԵ�����Ϊ4����EΪFeԪ�أ������Ų�ʽΪ[Ar]3d64s2���ݴ˷������
������������֪��AΪC��BΪN��CΪO��DΪAl��EΪFe��
(1)DΪAl��AlΪ13��Ԫ�أ������ڱ���λ��Ϊ�������ڣ���A�壻EΪFe����̬E2+�۵��ӵ��Ų�ͼΪ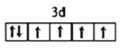 ��BΪN����������ߵĵ�������Ϊ2p������������ͣ��ʴ�Ϊ���������ڣ���A�壻
��BΪN����������ߵĵ�������Ϊ2p������������ͣ��ʴ�Ϊ���������ڣ���A�壻 �������ͣ�
�������ͣ�
(2)AΪC��CΪO����A��C�γɵ���ۻ�����ΪCO2������ԭ�ӵļ۲���Ӷ���=����ԭ�ӵ��������Ӷ���+�µ��Ӷ���=2+![]() =2��������ԭ�ӵĹ���ӻ�����Ϊsp�ӻ����ʴ�Ϊ��sp��
=2��������ԭ�ӵĹ���ӻ�����Ϊsp�ӻ����ʴ�Ϊ��sp��
(3)AΪC��BΪN��CΪO�����ֻ�̬ԭ�ӵĵ�һ�������ɴ�С��˳��ΪN��O��C��DΪAl��B��C��D�ļ����Ӻ�������Ų���ͬ���˵����Խ�뾶ԽС�������ǵİ뾶�ɴ�С��˳��Ϊr(N3-)��r(O2-)��r(Al3+)���ʴ�Ϊ��N��O��C��r(N3-)��r(O2-)��r(Al3+)��
(4)CΪO��O�ĺ�����18�����ӵ��⻯��ΪH2O2�������ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(5)EΪFe��Fe(CO)5���۵㡢�е�ϵͣ��������ڷ��Ӿ��壻�ʴ�Ϊ�����Ӿ��壻
(6)AΪC��BΪN���е�:N2H4>C2H6������N2H4���γɷ��Ӽ��������C2H6�����γɷ��Ӽ������ֻ�з��»�������˷е�ϵͣ��ʴ�Ϊ��ǰ�߿��γɷ��Ӽ����������ֻ�з��»�����
(7)һ��������Feԭ�ӵĸ���Ϊ![]() ����Nԭ�ӵĸ���Ϊ1���������Ļ�ѧʽΪFe4N��һ������������Ϊ
����Nԭ�ӵĸ���Ϊ1���������Ļ�ѧʽΪFe4N��һ������������Ϊ![]() ����þ�����ܶ�Ϊ
����þ�����ܶ�Ϊ![]() ���ʴ�Ϊ��Fe4N��
���ʴ�Ϊ��Fe4N��![]() ��
��

����Ŀ����һ���¶��£�������X������Y��0.16mol����10L�����ܱ������У�������Ӧ
X(g)��Y(g) ![]() 2Z(g) ��H < 0��һ��ʱ���ﵽƽ�⣬��Ӧ�����вⶨ���������±���
2Z(g) ��H < 0��һ��ʱ���ﵽƽ�⣬��Ӧ�����вⶨ���������±���
t/min | 2 | 4 | 7 | 9 |
n(Y)/mol | 0.12 | 0.11 | 0.10 | 0.10 |
����˵����ȷ����
A����Ӧǰ2min��ƽ�����ʦ�(Z)=2.0��10��3mol��L��1��min-1
B�������������䣬�����¶ȣ���Ӧ�ﵽ��ƽ��ǰ��(��)> ��(��)
C�����¶��´˷�Ӧ��ƽ�ⳣ��K=1.44
D�� �����������䣬�ٳ���0.2molZ��ƽ��ʱX�������������