��Ŀ����
18��ʵ��������һ�����ʵ���Ũ�ȵ���Һ���辭���㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ�Ȳ��裮��������0.2mol/L��CuSO4��Һ500mL���ش��������⣺
��1����������ƽ��ȡCuSO4•5H2O��������Ϊ250��������m=C��V��M=25.0g��
��2�����ܽⲢ��ȴ�����Һת�ƵĹ������õ��IJ��������в��������ձ���500ml����ƿ��
��3������ʱ����ˮ����̶���1-2cmʱ���ý�ͷ�ιܵμ�����ˮ����Һ�İ�Һ����̶������У�
��4�����������ʹ������ҺŨ��ƫ�͵���AB��������ţ�
A��δ��ϴ���ձ������Һת��������ƿ
B��������ˮʱ�����������˿̶���
C��������մ�����ʣ�����ʹ����������룩
D������ƿʹ��ǰδ�����
���� ��1������m=C��V��M�������ʵ�������
��2�������ܽ���Һ��Ҫʹ���ձ���ת��ʱ��Ҫ���Ų�����ת�Ƶ�500mL����ƿ�н��н��
��3������ʱ��Ҫ�ý�ͷ�ι����μӣ�
��4�������������������ʵ����ʵ�������Һ�������Ӱ�죬����C=$\frac{n}{V}$������������
��� �⣺��1����������ƽ��ȡCuSO4•5H2O��������Ϊ250��������m=C��V��M=0.2mol/L��0.5L��250g/mol=25.0g��
�ʴ�Ϊ��25.0g��
��2���ܽⲢ��ȴ�����Һת�ƵĹ������õ��IJ��������У�ʢ����Һ���ձ����������õIJ�������500mL����ƿ��
�ʴ�Ϊ�����������ձ���500ml����ƿ��
��3������ʱ��Ҫ�ý�ͷ�ι����μӣ�
�ʴ�Ϊ����ͷ�ιܣ�
��4��A��δ��ϴ���ձ������Һת��������ƿ���������ʵ����ʵ���ƫС����Һ��Ũ��ƫ�ͣ���Aѡ��
B��������ˮʱ�����������˿̶��ߣ�������Һ�����ƫ����ҺŨ��ƫ�ͣ���Bѡ��
C��������մ�����ʣ�����ʹ����������룩�����³�ȡ�����ʵ����ʵ���ƫ����Һ��Ũ��ƫ�ߣ���C��ѡ��
D������ƿʹ��ǰδ���������Һ����������ʵ����ʵ������������Ӱ�죬��ҺŨ�Ȳ��䣬��D��ѡ��
��ѡ��AB��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���Ϥ����ԭ�������ǽ���ؼ���ע������ƿ��ʹ��ע�������Ŀ�ѶȲ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ��ѧ��Ӧ��Ȼ���������仯 | |
| B�� | ��ѧ��Ӧ�е������仯��Ҫ���ɻ�ѧ���Ķ��Ѻ��γ������ | |
| C�� | ����������ķ�Ӧ�����Է���Ӧ | |
| D�� | Ҫ�жϷ�Ӧ���еķ������ۺϿ�����ϵ���ʱ���ر� |
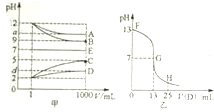 ����A��E�������±��е�������ɵģ������½������ʵ���Һ��1mLϡ�͵�1000mL��pH�ı仯��ϵ���ͼ��ʾ������A��D��Ӧ�õ�E����ش��������⣮
����A��E�������±��е�������ɵģ������½������ʵ���Һ��1mLϡ�͵�1000mL��pH�ı仯��ϵ���ͼ��ʾ������A��D��Ӧ�õ�E����ش��������⣮| ������ | NH4+��H+��Na+ |
| ������ | OH-��CH3COO-��Cl- |
��2��д��A��C��Ӧ�����ӷ���ʽ��NH3+H+=NH4+��
��3��ͼ��Ϊ����ʱ��25mLijŨ�ȵ�B��Һ����εμ�0.2mol��L-1��D��Һ�Ĺ�����pH�ı仯���ߣ�
��ͼ����B�����ʵ���Ũ��Ϊ0.1mol��L-1
��G����Һ�����ԣ������ǡ����ȫ��Ӧ�ĵ�����FG����FG��GH�����䣮
��G����Һ�и�����Ũ�ȴ�С��ϵ��c��Na+��=c��CH3COO-����c��H+��=c��OH-����
��4��t��ʱ��A��ϡ��Һ��c��H+��=10-amol��L-1��c��OH-��=10-bmol��L-1����֪a+b=13�����¶��£�t�棩����100mL0.2mol��L-1��C��Һ��100mL0.4mol��L-1��B��Һ��Ϻ���Һ����仯���Բ��ƣ�����Һ��pH=12��
| A�� | 20 | B�� | 50 | C�� | 0.05 | D�� | ��ȷ�� |
| A�� | K+ Na+ OH- SO42- | B�� | OH�� SO42- Al3+ Cl- | ||
| C�� | H+ Na+ CO32- Cl- | D�� | K+ Na+ NO3- Cu2+ |
N2��g��+3H2��g��?2NH3��g����H=-92.4kJ/mol �ݴ˻ش��������⣺
��1���ϳɰ���ҵ��ȡ�����д�ʩ�У���������ɳ����ԭ�����͵��Ǣڢۣ�����ţ���
�ٷ�ӦѹǿΪ20Mpa��50Mpa
��500��ĸ���
������ý������
�ܽ����ɵİ�Һ������ʱ����ϵ�з��������δ��Ӧ��N2��H2ѭ�����ϳ����У�
��2��һ��������NH3��ƽ�����������n��N2���仯��ͼ��ʾ��T-�¶ȣ���
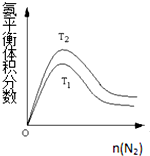
��T2T2��T1T1�����=���������жϵ������ǣ��ϳɰ���Ӧ�Ƿ��ȷ�Ӧ���¶����ߣ�ƽ�����淴Ӧ�����ƶ���NH3����������½�������T2��T1��
��3���ϳɰ������������������ˮú������õ����漰��Ӧ��Ϣ���£�
��Ӧһ��C��s��+H2O��g��?H2��g��+CO��g�� �� ƽ�ⳣ��K1
��Ӧ����CO��g��+H2O��g��?H2��g��+CO2��g�� ƽ�ⳣ��K2
��K1�ı���ʽ��K1=$\frac{c��H2��c��CO��}{c��H2O��}$��
�ڽ�һ������H2O��g����CO��g���ֱ�ͨ�뵽���Ϊ1L���ܱ������У��ڲ�ͬ�����½��з�Ӧ���õ������������ݣ�
| ʵ����� | �¶�/�� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | ||
| H2O | CO | H2 | CO | |||
| 1 | 650 | 1 | 2 | 0.8 | 1.2 | 5 |
| 2 | 900 | 0.5 | 1 | 0.2 | 0.8 | 3 |
| 3 | T | a | b | c | d | t |
������ʵ��3����a=0.5��b=1ʱ��Ҫʹc��d������ʵ��2��ͬ����t��3�����Բ�ȡ�Ĵ�ʩΪD������ţ�
A���������������¶�T��900��
B���������������¶�T��900��
C������һ��������
D��ʹ�ø�Ч����
�����ڷ�Ӧ�����������¶�ʱK2��С�����������С�����䡱����
| A�� | 18 gˮ�������ĵ�����Ϊ10NA | |
| B�� | �ڱ�״���£�1molˮ�����ԼΪ22.4L | |
| C�� | 0.3 mol•L-1Na2SO4��Һ�к�0.6NA��Na+ | |
| D�� | 11.2L�����к�NA����ԭ�� |
| A�� | ������Ϊ92 | B�� | ������Ϊ235 | C�� | ���������Ϊ143 | D�� | ������Ϊ92 |