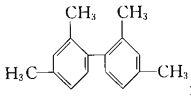
��Ŀ����
����Ŀ��ij������������ˮ��������Һ�п��ܺ���![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() �еļ������ӣ��Ҹ������ӵ����ʵ�����ͬ��Ϊ��ȷ������ɣ���������ʵ�飺
�еļ������ӣ��Ҹ������ӵ����ʵ�����ͬ��Ϊ��ȷ������ɣ���������ʵ�飺
(1)ȡһ��������Ʒ��������ˮ����ܽ⣬�õ�������Һ��
(2)ȡ������Һ����������ϡ���ᣬ�ٵ����������ᱵ��Һ���г������ɣ���������ã����ϲ���Һ�е�����������Һ���г������ɡ�
(3)��ȡ����![]() ����Һ����������������Һ���г������ɡ�
����Һ����������������Һ���г������ɡ�
��������ʵ�����ù�����Ʒ�ijɷֿ�����
A.�û����һ����NaCl��![]()
B.�û����һ����NaCl��![]()
C.�û���������![]() ��
��![]()
D.�û���������![]() ��
��![]() 3
3
���𰸡�D
��������
(1)��ȡһ��������Ʒ��������ˮ����ܽ⣬�õ�������Һ��֪����Һ��������![]() ��
��![]() �����ܴ���
�����ܴ���![]() ��
��
(2)��ȡ������Һ����������ϡ���ᣬ�ٵ����������ᱵ��Һ���г������ɣ�˵������![]() ���ӣ���������ã����ϲ���Һ�е�����������Һ���г������ɣ�����֤������
���ӣ���������ã����ϲ���Һ�е�����������Һ���г������ɣ�����֤������![]() ����Ϊ
����Ϊ![]() Ҳ�dz�����
Ҳ�dz�����
(3)��ȡ����(1)����Һ����������������Һ���г������ɣ�˵������![]() ��
��![]() ��
��![]() ��
��![]() ������
������![]() ��
��
���Ͽ�֪ ����Һ��һ������![]() ��һ������
��һ������![]() ��
��![]() ��
��![]() ��
��![]() ��������
��������![]() �����ܴ���
�����ܴ���![]() ��
��
A�����Һ�к���![]() ��
��![]() ��
��![]() ��
��![]() ��֪���û���ﲻ������NaCl��
��֪���û���ﲻ������NaCl��![]() ����A����
����A����
B��ɸ������ӵ����ʵ�����ͬ����Һ�к���![]() ��
��![]() ��
��![]() ��
��![]() ��֪���û���������NaCl��
��֪���û���������NaCl��![]() ����B����
����B����
C�����Һ�в���![]() ��֪���û���ﲻ������
��֪���û���ﲻ������![]() ��
��![]() ����C����
����C����
D��ɸ������ӵ����ʵ�����ͬ����Һ�к���![]() ��
��![]() ��
��![]() ��
��![]() ��֪���û���������
��֪���û���������![]() ��
��![]() ����D��ȷ��
����D��ȷ��
��ѡD��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ���Ͼ�Ӳ�ʺϽ��к�̼����(WC)��������(Co)�����������������õ�ⷨ����WC���Ʊ�Co2O3�Ĺ������̼�ͼ���£�
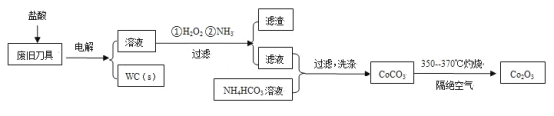
��֪�������������У����ֽ��������γ��������������pH��Χ���£�
�������� | Fe3+ | Fe2+ | Co2+ |
��ʼ������pH | 1.9 | 7.0 | 6.5 |
������ȫ��pH | 3.2 | 9.0 | 9.4 |
�ش��������⣺
��1���ԷϾɵ����������������������������Ϊ�������Һ�����ʱ�����ĵ缫��Ӧ�У�Co-2e-��Co2+��______��
��2��ͨ�백����Ŀ���ǵ�����Һ��pH����ȥ��Ԫ�ء��ɱ��е����ݿ�֪�������Ͽ�ѡ���pH�ķ�Χ��_______��
��3������CoCO3�����ӷ���ʽ��________��
��4��ʵ����NH4HCO3��Һ�Լ��ԡ��Ʊ�CoCO3ʱ�����ܽ���Һ����NH4HCO3��Һ�У�ԭ����_______��
��5����֪��Ksp(CoCO3)��1.4��10��13��Ksp(CoC2O4)��6.3��10��8�������ӳ���ת���Ƕȿ��ǣ���0.01mol/L Na2C2O4��Һ�м���CoCO3�����ܷ�ת��ΪCoC2O4������ͨ������˵����_________��
��6��ϴ��CoCO3����ֶ����ղ�Ʒ���Ȳ���Ӱ�죬���ڱ���ʱ����ɻ�����Ⱦ����Ҫԭ����____��
��7��CoCO3����Co2O3�Ļ�ѧ����ʽ��_________��
����Ŀ��ʵ�����Ʊ�1��2����������ķ�Ӧԭ��������ʾ��
��һ����CH3CH2OH![]() CH2=CH2��H2O��
CH2=CH2��H2O��
�ڶ�������ϩ����ˮ��Ӧ�õ�1��2���������顣
���ܴ��ڵ���Ҫ����Ӧ�У��Ҵ���Ũ����Ĵ�������140������ˮ��������(CH3CH2OCH2CH3)��������������������Ҵ��Ʊ�1��2�����������װ����ͼ��ʾ(����װ��δ����)��

�й������б����£�
�Ҵ� | 1��2���������� | ���� | |
״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
�ܶ�/(g/cm3) | 0.79 | 2.2 | 0.71 |
�е�/(��) | 78.5 | 132 | 34.6 |
�۵�/(��) | ��130 | 9 | ��116 |
��ش��������⣺
��1��д����ϩ����ˮ��Ӧ�Ļ�ѧ����ʽ��______��
��2���ڴ��Ʊ�ʵ���У�Ҫ������Ѹ�ٵذѷ�Ӧ�¶���ߵ�170�����ң�������ҪĿ����_____(����ĸ����)��
a��������Ӧ b���ӿ췴Ӧ����
c����ֹ�Ҵ��ӷ� d�����ٸ�������������
��3��װ��B��������______��
��4����װ��C��Ӧ����____ (����ĸ����)����Ŀ�������շ�Ӧ�п������ɵ�SO2��CO2���塣
a��ˮ b��Ũ���� c������������Һ d������̼��������Һ
��5����1��2����������ֲ�Ʒ���ڷ�Һ©���м�ˮ�����ã�����Ӧ��___ (����������������)�㡣
��6�������������������������ѣ�����____�ķ�����ȥ��