��Ŀ����
��֪CuSO4��Һ�ֱ���Na2CO3��Һ��Na2S��Һ�ķ�Ӧ������£�
��1��CuSO4+Na2CO3��Cu2++CO32��+H2O=Cu(OH)2��+CO2��
��2��CuSO4+Na2S�� Cu2++S2-=CuS��
���м������ʵ��ܽ�ȴ�С�ıȽ��У���ȷ����
��1��CuSO4+Na2CO3��Cu2++CO32��+H2O=Cu(OH)2��+CO2��
��2��CuSO4+Na2S�� Cu2++S2-=CuS��
���м������ʵ��ܽ�ȴ�С�ıȽ��У���ȷ����
| A��CuS��Cu(OH)2��CuCO3 | B��CuCO3��Cu(OH)2����uS |
| C��CuS��CuCO3��Cu(OH)2 | D��Cu(OH)2����uCO3��CuS |
A
�����������ѧ��Ӧ�Ĺ����������ܽ�ȴ��ת��Ϊ�ܽ��С�ġ��ӵ�һ����Ӧʽ�п��Եó��Ľ����ǣ��ܽ�ȣ�Cu(OH)2��CuCO3���ɵڶ�����ʽ���Եó��Ľ����ǣ�CuS��Cu(OH)2����Ϊ�ڵڶ�����Ӧ�У���Һ�м��������ӣ�Ҳ��ˮ��ֻ����������ͭ����û����������ͭ��������Ϊ��ͭ���ܽ�ȱ�������ͭ���ܽ��С�����ѡA��

��ϰ��ϵ�д�
�����Ŀ
 ��Cl������Һ����������AgNO3��Һʱ��
��Cl������Һ����������AgNO3��Һʱ��


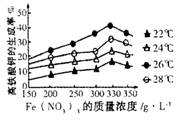


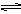 Ag+��aq�� + Cl-��aq����ƽ��ʱ��Ksp =C��Ag+ )��C(Cl-) ,�����Ȼ����ֱ�Ͷ���100 mLˮ ��24 mL 0��1 mol��L-1NaCl ��10 mL 0��1 mol��L-1MgCl2 ��30 mL 0��1 mol��L-1AgNO3��Һ��,��Һ��C(Ag+)�ɴ�С˳��Ϊ
Ag+��aq�� + Cl-��aq����ƽ��ʱ��Ksp =C��Ag+ )��C(Cl-) ,�����Ȼ����ֱ�Ͷ���100 mLˮ ��24 mL 0��1 mol��L-1NaCl ��10 mL 0��1 mol��L-1MgCl2 ��30 mL 0��1 mol��L-1AgNO3��Һ��,��Һ��C(Ag+)�ɴ�С˳��Ϊ