��Ŀ����
10������ʱ������˵������ȷ���ǣ�������| A�� | 0��l mol/L�������Һ�У�c��Ca2+����c��CH3COO-����c��OH-����c��H+�� | |
| B�� | ˮ���������c��H+��=10-13mol/L����Һ��K+��Cl-��NO3-��I-һ���ܴ������� | |
| C�� | ��0.1mol/LCH3COOH��Һ��ͨ������HCl������ĵ���ƽ�����淴Ӧ�����ƶ�������Һ��$\frac{c��C{H}_{3}COOH��}{c��{H}^{+}����c��C{H}_{3}CO{O}^{-}��}$���� | |
| D�� | �������ʵ���Ũ�ȵ�CH3COOH��CH3COONa�����Һ�У�2c��H+��-c��CH3COO-��=2c��OH-��-c��CH3COOH�� |
���� A�����������ˮ��̶Ƚ�С����c��CH3COO-����c��Ca2+����
B��ˮ���������c��H+��=10-13mol/L����ҺΪ���Ի������Һ������������������������ܹ����������ӣ�
C.$\frac{c��C{H}_{3}COOH��}{c��{H}^{+}����c��C{H}_{3}CO{O}^{-}��}$Ϊ����ĵ���ƽ�ⳣ���ĵ������¶Ȳ��䣬����ƽ�ⳣ�����䣬��ñ�ֵ���䣻
D�����ݻ��Һ�ĵ���غ㡢�����غ�����жϣ�
��� �⣺A���������Һ�У����ڴ��������ˮ��̶Ƚ�С����c��CH3COO-����c��Ca2+����������ȷ������Ũ�ȴ�СΪ��c��CH3COO-����c��Ca2+����c��OH-����c��H+������A����
B��ˮ���������c��H+��=10-13mol/L����Һ�д��ڴ��������ӻ����������ӣ�NO3-��I-�������������ܹ�����������ԭ��Ӧ������Һ�в��ܴ������棬��B����
C.$\frac{c��C{H}_{3}COOH��}{c��{H}^{+}����c��C{H}_{3}CO{O}^{-}��}$�����ƽ�ⳣ����Ϊ�����������¶Ȳ��䣬�����ƽ�ⳣ�����䣬���Ըñ�ֵ���䣬��C����
D���������ʵ���Ũ�ȵ�CH3COOH��CH3COONa�����Һ�У����ݵ���غ�ɵã�c��Na+��+c��H+��=c��CH3COO-��+c��OH-�������������غ�ɵã�2c��Na+��=c��CH3COO-��+c��CH3COOH�������߽�Ͽɵã�2c��H+��-c��CH3COO-��=2c��OH-��-c��CH3COOH������D��ȷ��
��ѡD��
���� ���⿼��������Ũ�ȴ�С�Ƚϡ����ӹ��桢������ʵĵ��뼰��Ӱ������أ���Ŀ�Ѷ��еȣ���ȷ�ε�ˮ��ԭ��������غ㼰�����غ�ĺ���Ϊ���ؼ���ע���������ӹ�����жϷ�����Ӱ��������ʵ�������أ�

| A�� | Cl | B�� | S | C�� | P | D�� | Si |
| A�� | 5.6 g Fe ��������HCl��ȫ��Ӧʧȥ������Ϊ0.3 NA | |
| B�� | 22.4L O2����NA��O2���� | |
| C�� | 0.2 mol/L CaCl2��Һ�к���Cl�����ӵ���ĿΪ0.4NA | |
| D�� | 1.6g CH4�����ĵ�����ΪNA |
| A�� | �������ƺ���ˮ��Ӧ��Na2O2+H2O�T2Na++2OH-+H2�� | |
| B�� | ����Ͷ�뵽NaOH��Һ�У�2Al+2OH-�T2AlO2-+H2�� | |
| C�� | ̼������ڴ��CaCO3+2H+�TCa2++CO2��+H2O | |
| D�� | �Ȼ�������Һ��ͨ��������2Fe2++Cl2�T2Fe3++2Cl- |
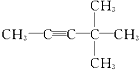
 ����ԭ����Ȳ�������ǣ�������
����ԭ����Ȳ�������ǣ�������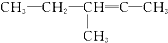

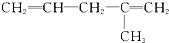
 ijͬѧ������װ�ã��̶����������������ԣ������йذ�����ȡ��ʵ��̽�����ش��������⣮
ijͬѧ������װ�ã��̶����������������ԣ������йذ�����ȡ��ʵ��̽�����ش��������⣮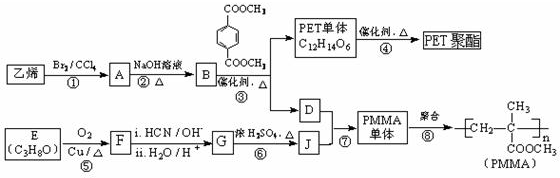
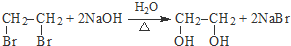 ��
��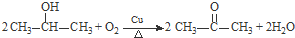 ��
��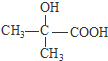 ��
�� ������NaOH��Һ��Ӧʱ���������4mol NaOH
������NaOH��Һ��Ӧʱ���������4mol NaOH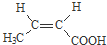 ��
�� ��
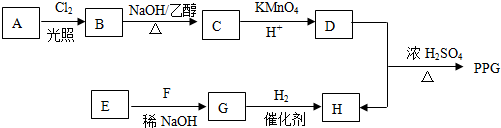
��
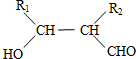
 ��
�� ��
��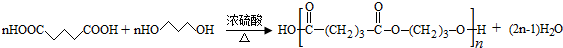 ��
�� ��д����ʽ��
��д����ʽ��
 ��
��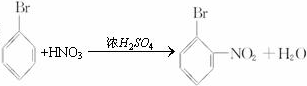 ��
�� ��
��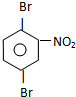 ��
��