��Ŀ����
8�����к�������NaCl��Na2SO4��Na2CO3�����ʵ�NaNO3��Һ��ѡ���ʵ����Լ���ȥ���ʣ��õ�������NaNO3���壬ʵ��������ͼ��ʾ��
��1������A����Ҫ�ɷ���BaSO4��BaCO3���ѧʽ����
��2�����з�Ӧ�����ӷ���ʽ��Ag++Cl-�TAgCl����
��3���٢ڢ��о����еķ�������ǹ��ˣ�
��4����Һ3�����������Եõ�NaNO3���壬��Һ3�п϶����е�������Na2CO3��Ϊ�˳�ȥ���ʣ�������Һ3�м���������HNO3��
��5��ʵ����������ʵ���õ�NaNO3��������500mL 0.40mol/L NaNO3��Һ��
��������Һʱ���������²�����a�����ݣ�b�����㣻c���ܽ⣻d��ҡ�ȣ�e��ת�ƣ�f��ϴ�ӣ�j����������ȡNaNO3�����������17.0 g�����ղ���˳��4����e������ţ���
��ijͬѧת����Һ�IJ�����ͼ��ʾ����ͬѧ�����еĴ�����δ�ò�����������
�����ý�ͷ�ιܶ���ʱ����С�ĵ�ˮ�ι��˿̶��ߣ�����ΪӦ�ò�ȡ�Ĵ��������ǣ��������ƣ�
�����в����У����������������Һ��Ũ��ƫ�͵���a����ѡ���
a��û��ϴ���ձ��Ͳ�����
b������ʱ�����ӿ̶���
c��ϴ�Ӻ������ƿ�в�����������ˮ��
���� ��ʵ�����̿�֪�����������Ba��NO3��2������BaSO4��BaCO3������Ȼ������Һ�м��������AgNO3��ʹCl-ȫ��ת��ΪAgCl��������������Һ�м��������Na2CO3��ʹ��Һ�е�Ag+��Ba2+��ȫ���������������ҺΪNaNO3��Na2CO3�Ļ�������ϡHNO3�����������������ɵù���NaNO3����������һ��Ũ�ȵ���Һ���������
��� �⣺��1�����������Ba��NO3��2��Na2SO4��Na2CO3��Ba��NO3��2��Ӧ����BaSO4��BaCO3�������ʴ�Ϊ��BaSO4��BaCO3��
��2����Һ1�к������ӣ����������AgNO3��ʹCl-ȫ��ת��ΪAgCl��������Ӧ�����ӷ���ʽΪ��Ag++Cl-�TAgCl�����ʴ�Ϊ��Ag++Cl-�TAgCl����
��3���٢ڢ��о����еķ����ǰѲ��������ʺͿ��������ʷ��룬��Ϊ���ˣ��ʴ�Ϊ�����ˣ�
��4����Һ3ΪNaNO3��Na2CO3�Ļ�������ϡHNO3���ɳ�ȥNa2CO3����������������ȴ�ᾧ�����˵õ������ƣ��ʴ�Ϊ��Na2CO3��HNO3��
��5�����Ʊ�500mL 0.4mol/L��NaNO3��Һ����Ҫ500mL����ƿ������Ҫ�����Ƶ�����Ϊ0.5L��0.4mol/L��85g/mol=17.0g�����ƵIJ�������Ϊ���㡢�������ܽ⡢ת�ơ�ϴ�ӡ����ݡ�ҡ�ȵȲ������ʵ��IJ�Ϊת�ƣ�
�ʴ�Ϊ��17.0��e��
������һ�����ʵ���Ũ�ȵ���Һ��Ҫ500mL����ƿ��������ƿ��ת��Һ����Ҫ�ò������������ʴ�Ϊ��δ�ò�����������
�����ý�ͷ�ιܶ���ʱ����С�ĵ�ˮ�ι��˿̶��ߣ�Ũ��ƫС�����������ƣ��ʴ�Ϊ���������ƣ�
��a��δϴ�Ӳ��������ձ�������ƫ�٣�Ũ��ƫ�ͣ�
b������ʱ���ӿ̶��ߣ����ƫС��Ũ��ƫ��
c������ƿ�в���ˮ�֣����ʵ����ʵ������䣬��Ũ����Ӱ�죬
�ʴ�Ϊ��a��
���� ���⿼�����ʵķ����ᴿ������һ�����ʵ���Ũ����Һ�����ƣ���Ŀ�Ѷ��еȣ�����ע��Cl-��SO42-�����ʣ����ճ���ԭ���ᴿʱ���������µ����ʣ�ע�����ʵ����Ⱥ�˳��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | 1 mol �����к���12.04��1023����ԭ�ӣ��ڱ�״����ռ�����22.4 L | |
| B�� | 1 mol������1.5 mol����������ͬ����ԭ���� | |
| C�� | �������Ũ�Ⱦ�Ϊ1 mol•L-1����������ᣬH3PO4��HCl���е���Ԫ������֮��Ϊ1��1 | |
| D�� | �����ʵ����ĸɱ��������ǣ�C6H12O6��������̼ԭ����֮��Ϊ1��6����ԭ����֮��Ϊ1��3 |
| A�� | Na��ˮ��Ӧ��Na+2H2O�TNa++2OH-+H2�� | |
| B�� | 0.01mol/L NH4Al��SO4��2��Һ��0.02mol/L Ba��OH��2��Һ�������� NH4++Al3++2SO42-+2Ba2++4 OH-�T2 Ba SO4��+Al��OH��3��+NH3•H2O | |
| C�� | ��������Һ�������Һ��ϣ�SiO32-+2H+�TH2SiO3�� | |
| D�� | Ũ�����м���������۲����ȣ�Fe+3NO3-+6H+ $\frac{\underline{\;\;��\;\;}}{\;}$Fe3++3NO2��+3H2O |
| A�� | 0.3 mol•L-1��Na2SO4��Һ�к���Na+��SO42-�������ʵ���Ϊ0.9 mol | |
| B�� | ��1 Lˮ����22.4 L����ʱ���ð�ˮ��Ũ�Ȳ���1 mol•L-1��ֻ�е�22.4 L��������ˮ�Ƶ�1 L��ˮʱ����Ũ�Ȳ���1 mol•L-1 | |
| C�� | ��K2SO4��NaCl�����Ի��ˮ��Һ�У����Na+��SO42-�����ʵ�����ȣ���K+��Cl-�����ʵ���Ũ��һ����ͬ | |
| D�� | 10��ʱ��100 mL 0.35 mol•L-1��KCl������Һ������5gˮ����ȴ��10��ʱ�������С��100 mL���������ʵ���Ũ����Ϊ0.35 mol•L-1 |
| A�� | �����ɳ����ﵽ�����ʱ������NaOH��Һ�����Ϊ150mL | |
| B�� | ������ȫ���ܽ�ʱ�ռ���NO��������Ϊ4.48L����״���£� | |
| C�� | �μӷ�Ӧ�Ľ�����������һ����9.9g | |
| D�� | ������ȫ���ܽ�ʱ���μӷ�Ӧ����������ʵ���һ����0.6mol |
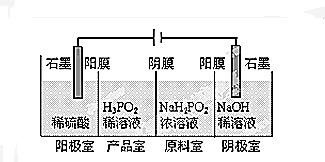

 ��ͼ�����ʽ�ζ��ܺ��ձ��зֱ�ע��0.2mol•L-1Ba��OH��2��Һ��0.1mol•L-1ϡ�����50mL������ϡ�����еμӼ���ʯ����Һ������ͼװ�����Ӻã�
��ͼ�����ʽ�ζ��ܺ��ձ��зֱ�ע��0.2mol•L-1Ba��OH��2��Һ��0.1mol•L-1ϡ�����50mL������ϡ�����еμӼ���ʯ����Һ������ͼװ�����Ӻã�