��Ŀ����
����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������A��Dͬ���壬C��Eͬ���壬D��E��Fͬ���ڣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C�γɵ���������ȵķ��ӣ���A��C�γɵĻ����ﳣ����ΪҺ̬��A�ֱܷ���E��F�γɵ���������ȵ�������ӣ�
��ش��������⣨���е���ĸֻ����Ԫ�ش��ţ���ʵ��Ԫ�ط����أ���
��1��A��F����Ԫ��ԭ�ӣ�ԭ�Ӱ뾶������
��2��A��C��D����Ԫ����ɵ�һ�ֳ������������Ҫ�Ĺ�ҵ��Ʒ���û��������ʽΪ��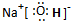
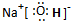 ��
��
��3��B��F����Ԫ���γɵ�һ�ֻ�������ӣ���ԭ�Ӿ���˵��ӽṹ������B�Ը��ۣ�F�����ۣ���û�����ˮ�����Ҫ�����ǣ�
��4��A��C��E����Ԫ���γɵ�һ�ֳ����������Ũ��Һ�ڼ��������¿���ͭ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ
��ش��������⣨���е���ĸֻ����Ԫ�ش��ţ���ʵ��Ԫ�ط����أ���
��1��A��F����Ԫ��ԭ�ӣ�ԭ�Ӱ뾶������
D
D
������ţ�����2��A��C��D����Ԫ����ɵ�һ�ֳ������������Ҫ�Ĺ�ҵ��Ʒ���û��������ʽΪ��
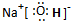
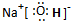
��3��B��F����Ԫ���γɵ�һ�ֻ�������ӣ���ԭ�Ӿ���˵��ӽṹ������B�Ը��ۣ�F�����ۣ���û�����ˮ�����Ҫ�����ǣ�
HClO��NH3��NH3?H2O��
HClO��NH3��NH3?H2O��
����4��A��C��E����Ԫ���γɵ�һ�ֳ����������Ũ��Һ�ڼ��������¿���ͭ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ
Cu+2H2SO4��Ũ��
CuSO4+SO2��+2H2O
| ||
Cu+2H2SO4��Ũ��
CuSO4+SO2��+2H2O
��
| ||
������A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������A��C�γɵĻ����ﳣ����ΪҺ̬��������Ϊˮ����AΪHԪ�أ�CΪOԪ�أ�A��B������������֮����C��������������ȣ�B������������Ϊ5����BΪNԪ�أ�A��Dͬ���壬��DΪNaԪ�أ�C��Eͬ���壬��EΪSԪ�أ�D��E��Fͬ���ڣ�A�ֱܷ���E��F�γɵ���������ȵ�������ӣ���FΪClԪ�أ�
��1��ͬ������ԭ����������ԭ�Ӱ뾶��С��������������ͬ�����Ӳ�Խ��ԭ�Ӱ뾶Խ��
��2��NaOH���������ӻ�����������������������ӹ��ɣ�
��3�����������γɵĻ���������Ƴ��������γɵĸ�ԭ�Ӿ���˵��ӽṹ�Ļ�������NCl3�������е��ʸ��ۣ��ȳ����ۣ�������ˮ�����Ҫ����ΪHClO��NH3��NH3?H2O����
��4��Ũ������ͭ��Ӧ����������ͭ������������ˮ��
��1��ͬ������ԭ����������ԭ�Ӱ뾶��С��������������ͬ�����Ӳ�Խ��ԭ�Ӱ뾶Խ��
��2��NaOH���������ӻ�����������������������ӹ��ɣ�
��3�����������γɵĻ���������Ƴ��������γɵĸ�ԭ�Ӿ���˵��ӽṹ�Ļ�������NCl3�������е��ʸ��ۣ��ȳ����ۣ�������ˮ�����Ҫ����ΪHClO��NH3��NH3?H2O����
��4��Ũ������ͭ��Ӧ����������ͭ������������ˮ��
����⣺A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������A��C�γɵĻ����ﳣ����ΪҺ̬��������Ϊˮ����AΪHԪ�أ�CΪOԪ�أ�A��B������������֮����C��������������ȣ�B������������Ϊ5����BΪNԪ�أ�A��Dͬ���壬��DΪNaԪ�أ�C��Eͬ���壬��EΪSԪ�أ�D��E��Fͬ���ڣ�A�ֱܷ���E��F�γɵ���������ȵ�������ӣ���FΪClԪ�أ���AΪHԪ�أ�BΪNԪ�أ�CΪOԪ�أ�DΪNaԪ�أ�EΪSԪ�أ�FΪClԪ�أ�
��1��ͬ������ԭ����������ԭ�Ӱ뾶��С��������������ͬ�����Ӳ�Խ��ԭ�Ӱ뾶Խ������Naԭ�Ӱ뾶��ʴ�Ϊ��D��
��2��AΪHԪ�أ�CΪOԪ�أ�DΪNaԪ�أ���Ԫ���γɵij���������ΪNaOH���������ӻ�����������������������ӹ��ɣ�����ʽΪ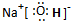 ��
��
�ʴ�Ϊ��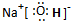 ��
��
��3��BΪNԪ�أ�FΪClԪ�أ�����Ԫ���γɵ�һ�ֻ�������ӣ���ԭ�Ӿ���˵��ӽṹ���û�������NCl3������N�Ը��ۣ�Cl�����ۣ���ˮ�����Ҫ����ΪHClO��NH3��NH3?H2O����
�ʴ�Ϊ��HClO��NH3��NH3?H2O����
��4��AΪHԪ�أ�CΪOԪ�أ�EΪSԪ�أ�����Ԫ���γɵ�һ�ֳ����������Ũ��Һ�ڼ��������¿���ͭ��Ӧ���û�����ΪH2SO4���÷�Ӧ�Ļ�ѧ����ʽΪCu+2H2SO4��Ũ��
CuSO4+SO2��+2H2O��
�ʴ�Ϊ��Cu+2H2SO4��Ũ��
CuSO4+SO2��+2H2O��
��1��ͬ������ԭ����������ԭ�Ӱ뾶��С��������������ͬ�����Ӳ�Խ��ԭ�Ӱ뾶Խ������Naԭ�Ӱ뾶��ʴ�Ϊ��D��
��2��AΪHԪ�أ�CΪOԪ�أ�DΪNaԪ�أ���Ԫ���γɵij���������ΪNaOH���������ӻ�����������������������ӹ��ɣ�����ʽΪ
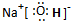 ��
���ʴ�Ϊ��
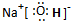 ��
����3��BΪNԪ�أ�FΪClԪ�أ�����Ԫ���γɵ�һ�ֻ�������ӣ���ԭ�Ӿ���˵��ӽṹ���û�������NCl3������N�Ը��ۣ�Cl�����ۣ���ˮ�����Ҫ����ΪHClO��NH3��NH3?H2O����
�ʴ�Ϊ��HClO��NH3��NH3?H2O����
��4��AΪHԪ�أ�CΪOԪ�أ�EΪSԪ�أ�����Ԫ���γɵ�һ�ֳ����������Ũ��Һ�ڼ��������¿���ͭ��Ӧ���û�����ΪH2SO4���÷�Ӧ�Ļ�ѧ����ʽΪCu+2H2SO4��Ũ��
| ||
�ʴ�Ϊ��Cu+2H2SO4��Ũ��
| ||
���������⿼��λ�á��ṹ�����ʵĹ�ϵ��Ӧ�ã��ۺ��Խ�ǿ������֪ʶ��϶࣬Ԫ�ص��ƶ��ǽ����Ĺؼ���A��C�γɵĻ����ﳣ����ΪҺ̬���ƶ�ͻ�ƿڣ�����ϤԪ�ػ����������������ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
 ����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��
����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��






