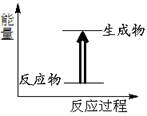
��Ŀ����
(10��)���ǵ����Ϻ����ḻ��ԭ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���á�
(1)25��ʱ��0.1mol/LNH4NO3��Һ��ˮ�ĵ���̶� ������ڡ��������ڡ���С�ڡ��� 0.1mol/L NaOH��Һ��ˮ�ĵ���̶ȡ�
(2)����0.1mol/L NaOH��Һ��0.2mol/LNH4NO3��Һ�������ϣ������Һ��
2c(NH4+)��c(NO3��)��������Һ������Ũ���ɴ�С��˳���� ��
(3)������ʱ��(N2H4)Ϊȼ�ϣ����������������������߷�Ӧ���ɵ�������̬ˮ�����ⶨ16g������������Ӧ�зų�284kJ��������
��÷�Ӧ���Ȼ�ѧ����ʽ�� ��
(4)��ͼ��1mol NO2��1mol CO��Ӧ����CO2��NO�����������仯ʾ��ͼ��
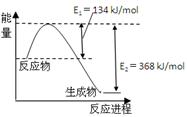
��֪��N2(g)��O2(g)��2NO(g) ��H����180kJ/mol
2NO (g)��O2(g)��N2(g)��2CO2(g) ��H����112.3kJ/mol
��Ӧ��2NO(g)��CO(g) N2(g)��2CO2(g)����H�� ��
N2(g)��2CO2(g)����H�� ��
(1)25��ʱ��0.1mol/LNH4NO3��Һ��ˮ�ĵ���̶� ������ڡ��������ڡ���С�ڡ��� 0.1mol/L NaOH��Һ��ˮ�ĵ���̶ȡ�
(2)����0.1mol/L NaOH��Һ��0.2mol/LNH4NO3��Һ�������ϣ������Һ��
2c(NH4+)��c(NO3��)��������Һ������Ũ���ɴ�С��˳���� ��
(3)������ʱ��(N2H4)Ϊȼ�ϣ����������������������߷�Ӧ���ɵ�������̬ˮ�����ⶨ16g������������Ӧ�зų�284kJ��������
��÷�Ӧ���Ȼ�ѧ����ʽ�� ��
(4)��ͼ��1mol NO2��1mol CO��Ӧ����CO2��NO�����������仯ʾ��ͼ��

��֪��N2(g)��O2(g)��2NO(g) ��H����180kJ/mol
2NO (g)��O2(g)��N2(g)��2CO2(g) ��H����112.3kJ/mol
��Ӧ��2NO(g)��CO(g)
 N2(g)��2CO2(g)����H�� ��
N2(g)��2CO2(g)����H�� ����1������
��2��c(NO3��)��c(NH4+)��c(Na+)��c(OH��)��c(H+)
��3��2N2H4(g)��2NO2(g)��3N2(g)��2H2O (g) ��H����1136kJ/mol
��4����760.3kJ/mol
��2��c(NO3��)��c(NH4+)��c(Na+)��c(OH��)��c(H+)
��3��2N2H4(g)��2NO2(g)��3N2(g)��2H2O (g) ��H����1136kJ/mol
��4����760.3kJ/mol
���������(1)NH4+��ˮ�⣬�ٽ���ˮ�ĵ��룬��OH��������ˮ�ĵ��룬��ˣ�0.1mol/L
NH4NO3��Һ��ˮ�ĵ���̶ȴ���0.1mol/L NaOH��Һ��ˮ�ĵ���̶ȡ�
(2)����Һ��Ϻ�������Һ������ΪNaNO3��NH3��H2O��NH4NO3������Һ��2c(NH4+)��c(NO3��)����NH3��H2O�ĵ������NH4+��ˮ�⣬�����Һ�ʼ��ԣ��ʸ����ӵ�Ũ�ȴ�СΪ
c(NO3��)��c(NH4+)��c(Na+)��c(OH��)��c(H+)
(3)N2H4��NO2��Ӧ�Ļ�ѧ����ʽΪ��2N2H4+2NO2=3N2+4H2O����֪16g����0.5mol��������������Ӧ�зų�284kJ������������2mol�����巴Ӧ�ų�������Ϊx���ɵõ�ʽ��
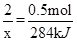 x=1136kJ�����ԣ����Ȼ�ѧ����ʽΪ2N2H4(g)��2NO2(g)��3N2(g)��2H2O (g) ��H����1136kJ/mol
x=1136kJ�����ԣ����Ȼ�ѧ����ʽΪ2N2H4(g)��2NO2(g)��3N2(g)��2H2O (g) ��H����1136kJ/mol(4)���ݸ�˹���ɣ��ɼ�����ô˷�Ӧ����H=��760.3kJ/mol
������������Ҫ�����˵������Һ������Ũ�ȴ�С�ıȽϡ��Ȼ�ѧ����ʽ����д�����ݡ�
������Һ������Ũ�ȴ�С�ıȽϣ����ֿ�����Һ�����ӵ�ˮ������룻
�����Ȼ�ѧ����ʽ����д��Ӧע������ͨ��ѧ����ʽ��д������ͬʱ��Ӧ�ȵļ�����ҪӦ�ø�˹���ɡ�

��ϰ��ϵ�д�
�����Ŀ
 2CO(g)��
2CO(g)�� O2(g)===CO(g)����H2
O2(g)===CO(g)����H2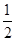 O2(g)===H2O(l)����H����285.8 kJ��mol��1 ����H2��ȼ����Ϊ��285.8 kJ��mol��1
O2(g)===H2O(l)����H����285.8 kJ��mol��1 ����H2��ȼ����Ϊ��285.8 kJ��mol��1 O2������=CO�������� ��H ="-393.5" kJ/mol
O2������=CO�������� ��H ="-393.5" kJ/mol