��Ŀ����
15�����̿���Ҫ�ɷ�ΪMnO2������������������ͭ�����Ƚ��������������������ѳ�ȼúβ���е�SO2�����Ƶõ�ز���MnO2����Ӧ������ʡ�ԣ���������ͼ���£���ش��������⣺
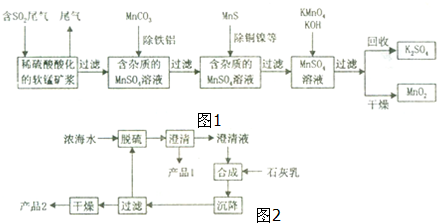
��1����������������ʵ��B��ѡ��������ĸ��ţ���
A����������ۺ����á�������B����ɫ��Ⱦ�ļ��١�������C������ļ��� D�����չ��ؽ���
��2��MnCO3������ˮ��ȴ�ܳ�ȥ��Һ��Al3+��Fe3+����ԭ����������Һ�е��ᣬ�ٽ�Al3+��Fe3+ˮ�������������������
��3����������м���KMnO4��ȡ��ƷMnSO2�Ļ�ѧ����ʽ��2KMnO4+3MnSO4+4KOH=5MnO2+3K2SO4+2H2O��
��4����ˮ��ȡþ��������Ҳ�õ�������ȥ��������ӣ�������������ͼ2���ù��չ����У��������Ҫ��ӳ�����ӷ���ʽΪCa2++SO42-�TCaSO4������Ʒ2�Ļ�ѧʽΪMg��OH��2��ʯ����������ص����ṩCa2+ʹ��ˮ�����ṩOH-ʹ��ˮ�е�Mg2+����Mg��OH��2������
��5����ͨп�̵�طŵ�ʱ��������Ҫ��ӦΪ��Zn+2NH4Cl+2MnO2�TZn��NH3��2Cl2+2MnOOH���������ĵ缫��Ӧʽ��MnO2+e-+NH4+�TMnOOH+NH3��
���� ������������������̷�Ӧ���������̣���MnCO3�ܳ�ȥ��Һ��Al3+��Fe3+��MnS��ͭ�������ӻ�ԭΪ���ʣ�����������������̷�Ӧ���ɶ������̣�ͨ�����˻�ö������̣�
��1��SO2���γ����꣬�������ʵ���˷�������ۺ����ã�ͬʱҲ�����������γɣ�ͬʱ���չ��ؽ���ͭ������
��2����������Һ�е��ᣬ�ٽ�Al3+��Fe3+ˮ��Ƕȷ�����
��3����������м���KMnO4��MnSO2����������ԭ�����ɶ������̣�
��4����ˮ�������ø������������������������������ƶ���ȥ����ʯ��ʯ�����ṩ��������������þ���ӽ�ϳ����ܵ�������þ��
��5������ԭ��ع���ԭ���ж�����������ԭ��Ӧ��Ȼ������ܷ�Ӧд�������ĵ缫��Ӧʽ��
��� �⣺������������������̷�Ӧ���������̣���MnCO3�ܳ�ȥ��Һ��Al3+��Fe3+��MnS��ͭ�������ӻ�ԭΪ���ʣ�����������������̷�Ӧ���ɶ������̣�ͨ�����˻�ö������̣�
��1����ɫ��Ⱦ��Ҫ�����ϵ��ѽ���������γɵģ�SO2���γ����꣬�������ʵ���˷�������ۺ����ã�ͬʱҲ�����������γɣ�ͬʱ���չ��ؽ���ͭ����������B���ϣ�
�ʴ�Ϊ��B��
��2������̼������������Һ�е��ᣬ������Һ�����ԣ��Ӷ��ٽ�Al3+��Fe3+ˮ��������������������ʴ�Ϊ��������Һ�е��ᣬ�ٽ�Al3+��Fe3+ˮ�������������������
��3��KMnO4��Һ���뵽MnSO4��Һ���Ʊ�MnO2��2�������ӱ�����������������ɶ������̣���Ӧ�Ļ�ѧ����ʽΪ��2KMnO4+3MnSO4+4KOH=5MnO2+3K2SO4+2H2O��
�ʴ�Ϊ��2KMnO4+3MnSO4+4KOH=5MnO2+3K2SO4+2H2O��
��4����ˮ�������ø������������������������������ƶ���ȥ����Ӧ�����ӷ���ʽΪ��Ca2++SO42-=CaSO4������ʯ��ʯ�����ṩ��������������þ���ӽ�ϳ����ܵ�������þ���ʴ�Ϊ��Ca2++SO42-�TCaSO4����Mg��OH��2���ṩCa2+ʹ��ˮ�����ṩOH-ʹ��ˮ�е�Mg2+����Mg��OH��2������
��5��ԭ����и���ʧȥ���ӣ������õ����ӣ���˼���п�̵�طŵ�ʱ�������Ƕ������̵õ����ӣ���缫��ӦʽΪ��MnO2+e-+NH4+�TMnOOH+NH3���ʴ�Ϊ��MnO2+e-+NH4+�TMnOOH+NH3��
���� ����ͨ���������̵��Ʊ������������ʵķ��롢�ᴿ���������ۺ�Ӧ�ã���Ŀ�Ѷ��еȣ���ȷ�Ʊ�ԭ��Ϊ���ؼ���ע�����ջ�ѧʵ������������������ʵķ��롢�ᴿ������������������ѧ���ķ�����������������ѧʵ��������

 ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д� �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д� Сѧ�����ҵ���ϴ�ѧ������ϵ�д�
Сѧ�����ҵ���ϴ�ѧ������ϵ�д� ���Ž�����ٰθ��νӹ㶫���������ϵ�д�
���Ž�����ٰθ��νӹ㶫���������ϵ�д� �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д�| A�� | ������ۡ��е�ߣ�Ӳ�ȴ� | |
| B�� | �����ʵĻ�ѧʽΪCO4 | |
| C�� | ������Cԭ������C-O��ѧ����֮��Ϊ1��4 | |
| D�� | ����Ŀռ���С����12��ԭ�ӹ��� |
 W��M��X��Y��Z���ֶ�����Ԫ�������ڱ��е�λ�������ʾ������ֻ��MΪ����Ԫ�أ���ش��������⣺
W��M��X��Y��Z���ֶ�����Ԫ�������ڱ��е�λ�������ʾ������ֻ��MΪ����Ԫ�أ���ش��������⣺| W | ||||
| M | X | Y | Z |
 ���Ƚ�Y��Z����Ԫ�صļ����ӵİ뾶��С��S2-��Cl-�������ӷ��ű�ʾ��
���Ƚ�Y��Z����Ԫ�صļ����ӵİ뾶��С��S2-��Cl-�������ӷ��ű�ʾ����2��д��X����������W���⻯���ˮ��Һ��Ӧ�Ļ�ѧ����ʽ��SiO2+4HF=SiF4��+2H2O��
��3����ѧ�����Ƴ�һ��Ӣ����Ϊsulflower�����ͷ���C16Y8���ṹ��ͼ��ʾ����1mol C16Y8�������8mol H2�����ӳɷ�Ӧ��
��4������ʯ��һ��������������ΪM2[XO4]WOH��������ǿ����Һ�лᷢ����ʴ��д������ʯ��ĩ�����NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��Al2[SiO4]FOH+5NaOH=2NaAlO2+Na2SiO3+NaF+3H2O��
| A�� | ��ˮ���ձ��ڱڻ�������Ũ�����У����ò��������Ͻ��� | |
| B�� | ʵ������ȡ����ʱ��β���ü�Һ���� | |
| C�� | ȡ�ý���������ʵ���ʣ���ҩƷҪ�Ż�ԭƿ | |
| D�� | ������ʱ�����������ȶ�������������Ʈ������ |
| A�� | ��SO2Ư��ʳƷ | B�� | �ù�����̫���ܵ�� | ||
| C�� | ��ҵ���õ����������ϳɰ� | D�� | ���������ʴ�̲��� |
| A�� | ���³�ѹ�£�32gO2�к��е������Ӹ���Ϊ2NA | |
| B�� | 1Ħ��CH4����������ĿΪ10NA | |
| C�� | ��״����22.4LH2O����������ĿΪNA | |
| D�� | 0.1 mol/L ϡ�����к���H+����Ϊ0.1 NA |
��Al��OH��3 ��Al2O3 �ۣ�NH4��2CO3 ��SiO2��
| A�� | �٢ڢ� | B�� | �ڢۢ� | C�� | �٢ڢ� | D�� | �٢ڢۢ� |

| A�� | ���ڿ�����ȼ��ʱ���������Ļ��棬�����к��� | |
| B�� | SO2ʹ��ˮ��ɫ����ʹKMnO4��Һ��ɫ��ԭ����ͬ | |
| C�� | ���е�̼̼���ļн�Ϊ109��28�� | |
| D�� | ����ϡ���������¿������ᷢ��ȡ����Ӧ������̼���� |
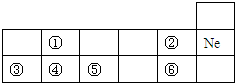 ��ͼ�����ڱ��е�һ���֣�
��ͼ�����ڱ��е�һ���֣� ��
��