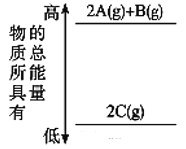
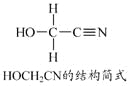
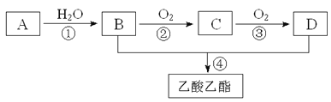
��Ŀ����
����Ŀ��̼Ԫ�ز������γɷḻ��ʵ��л���������һ����γɶ�������������C��ͬʱ���������γɶ��ֵ�����D��E��̼���仯�������;�㷺��
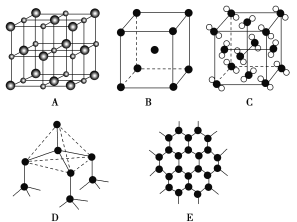
��֪AΪ���Ӿ��壬BΪ�������壬CΪ���Ӿ���
(1)ͼ�зֱ���������ֳ����ľ��壬�ֱ��ǣ�A________��B________��C________��D________��E________��(�����ƻ�ѧʽ)
(2)�ɱ��ͱ������ֳ����ķ��Ӿ��壬�������־���ıȽ�����ȷ����_____��
a.������ܶȣ��ɱ�>�� b.������۵㣺�ɱ�>��
c.�����еĿռ������ʣ��ɱ�>�� d.�����з��Ӽ��������������ͬ
(3) ���ʯ��ʯī��̼�����ֳ������ʣ�����������ȷ����________��
a.���ʯ��̼ԭ�ӵ��ӻ�����Ϊsp3�ӻ���ʯī��̼ԭ�ӵ��ӻ�����Ϊsp2�ӻ�
b.�����й��ۼ��ļ��������ʯ��C��C<ʯī��C��C
c.������۵㣺���ʯ>ʯī
d.�����й��ۼ��ļ��ǣ����ʯ>ʯī
e.���ʯ������ֻ���ڹ��ۼ���ʯī����������ڹ��ۼ����������ͷ��»���
f.���ʯ��ʯī���۵㶼�ܸߣ����Խ��ʯ��ʯī����ԭ�Ӿ���
(4)���ʯ�����ṹ��ͼ��һ�������е�Cԭ����ĿΪ ________��
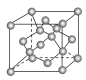
(5)C���ȸʯ���ȿ��Եõ�����ͭ������ͭ���������������ܶѻ�����֪Cu���ʵľ����ܶ�Ϊ�� g/cm3��Cu�����ԭ������ΪM�������ӵ�����ΪNA����Cu��ԭ�Ӱ뾶Ϊ __________cm��
���𰸡�NaCl Na �ɱ� ���ʯ ʯī ac ae 8 ![]() ��
��![]() cm
cm
��������
��1������ͼ�о���Ľṹ����ϳ��������֪��AΪ�Ȼ��ơ�BΪNa��CΪ�ɱ���DΪ���ʯ��EΪʯī��
��2��ˮ���Ӽ���������������з����ԣ�����ˮ�����γɱ�ʱ���ڽϴ�Ŀ�϶���������ڻ�ʱ������ƣ��ɱ�����֮��ֻ���ڷ��»������γɵķ��Ӿ������ܶѻ���
��3��a�����ʯ��̼ԭ�����ĸ�̼ԭ���γ�4�����۵����������������壬ʯī�е�̼ԭ�������ڵ�����̼ԭ���ԦҼ���ϣ��γ�ƽ���������νṹ��
b��c��sp2�ӻ��У�s����ijɷֱ�sp3�ӻ����࣬����ʯī��̼ԭ�ӻ��д�м������γɵĹ��ۼ����̣����ι̣���ʯī�IJ��ڹ��ۼ������Ƚ��ʯ�ļ����̣������������ƻ���ѧ����Ҫ����������
d�����ʯ��̼ԭ�����ĸ�̼ԭ���γ�4�����۵����������������壬ʯī�е�̼ԭ����sp2�ӻ���������ڵ�����̼ԭ���ԦҼ���ϣ��γ��������ε�ƽ���״�ṹ��
e�����ʯ��̼ԭ�����ĸ�̼ԭ���γ�4�����۵����������������壬������ֻ���й��ۼ���ʯī�е�̼ԭ����sp2�ӻ���������ڵ�����̼ԭ���ԦҼ���ϣ��γ��������ε�ƽ���״�ṹ����ÿ��̼ԭ�ӻ���һ��2p�����������һ��2p���ӣ���Щp����ֶ�����ƽ�У�����ֱ��̼ԭ��sp2�ӻ�������ɵ�ƽ�棬�γ��˴�м��������Щ�е��ӿ���������̼ԭ��ƽ���ϻ�����ƽ����������ʣ�ʯīΪ��״�ṹ�������֮��ͨ�����»������ӣ�
f��ʯīΪ��״�ṹ�������֮��ͨ�����»������ӣ�
��4���ɽ��ʯ�ľ����ṹ��֪�������ڲ���4��Cԭ�ӣ���������6��Cԭ�ӣ�������8��Cԭ�ӣ����ݾ�̯�����㣻
��5������ͭ���������������ܶѻ������þ�̯�����㾧��ԭ����Ŀ��ͭԭ�Ӱ뾶Ϊrcm���ɼ��㾧������������m=��V����ͭԭ�Ӱ뾶��
��
��1������ͼ�о���Ľṹ����ϳ��������֪��AΪ�Ȼ��ơ�BΪNa��CΪ�ɱ���DΪ���ʯ��EΪʯī��
��Ϊ��NaCl��Na���ɱ������ʯ��ʯī��
��2��a��ˮ���Ӽ���������������з����ԣ�����ˮ�����γɱ�ʱ���ڽϴ�Ŀ�϶���ܶȱ�ˮС���ɱ�����֮��ֻ���ڷ��»������γɵķ��Ӿ������ܶѻ����ܶȱ�ˮ��a��ȷ��
b�����ڻ�ʱ������ƣ��ɱ�����֮��ֻ���ڷ��»������ڻ�ʱ�ƻ����»���������ȷ��»���ǿ���ʾ�����۵�����ɱ�����b����
c��ˮ���Ӽ���������������з����ԣ�����ˮ�����γɱ�ʱ���ڽϴ�Ŀ�϶���ɱ�����֮��ֻ���ڷ��»������γɵķ��Ӿ������ܶѻ��������еĿռ������ʣ��ɱ���������c��ȷ��
d���ɱ�����֮��ֻ���ڷ��»�����ˮ����֮��ȴ��ڷ��»����ִ�������������з��Ӽ�����������Ͳ���ͬ����d����
��ѡ��ac��
��3��a�����ʯ��̼ԭ�����ĸ�̼ԭ���γ�4�����۵����������������壬̼ԭ�ӵ��ӻ�����Ϊsp3�ӻ���ʯī�е�̼ԭ�������ڵ�����̼ԭ���ԦҼ���ϣ��γ�ƽ���������νṹ��̼ԭ�ӵ��ӻ�����Ϊsp2�ӻ�����a��ȷ��
b��sp2�ӻ��У�s����ijɷֱ�sp3�ӻ����࣬����ʯī��̼ԭ�ӻ��д�м������γɵĹ��ۼ����̣����ι̣���ʯī�IJ��ڹ��ۼ������Ƚ��ʯ�ļ����̣���b����
c��ʯī�IJ��ڹ��ۼ������Ƚ��ʯ�ļ����̣������������ƻ���ѧ����Ҫ�������������Ծ�����۵���ʯ��ʯī����c����
d�����ʯ��̼ԭ�����ĸ�̼ԭ���γ�4�����۵����������������壬����Ϊ109��28�䣬ʯī�е�̼ԭ����sp2�ӻ���������ڵ�����̼ԭ���ԦҼ���ϣ��γ��������ε�ƽ���״�ṹ������Ϊ120�㣬��d����
e�����ʯ��̼ԭ�����ĸ�̼ԭ���γ�4�����۵����������������壬ʯī�е�̼ԭ����sp2�ӻ���������ڵ�����̼ԭ���ԦҼ���ϣ��γ��������ε�ƽ���״�ṹ����ÿ��̼ԭ�ӻ���һ��2p�����������һ��2p���ӣ���Щp����ֶ�����ƽ�У�����ֱ��̼ԭ��sp2�ӻ�������ɵ�ƽ�棬�γ��˴�м��������Щ�е��ӿ���������̼ԭ��ƽ���ϻ�����ƽ����������ʣ�ʯīΪ��״�ṹ�������֮��ͨ�����»������ӣ�˵�������к��й��ۼ��������������»�������e��ȷ��
f�����ʯ��ԭ�Ӿ��壬ʯīΪ��״�ṹ�������֮��ͨ�����»������ӣ�ʯīΪ����;��壬������ԭ�Ӿ��壬��f����
��ѡ��ae��
��4���ɽ��ʯ�ľ����ṹ��֪�������ڲ���4��Cԭ�ӣ���������6��Cԭ�ӣ�������8��Cԭ�ӣ����Խ��ʯ������cԭ����ĿΪ4+6��![]() +8��
+8��![]() =8��
=8��
����8��
��5������ͭ���������������ܶѻ���������Cuԭ����ĿΪ8��![]() +6��
+6��![]() =4��ͭԭ�ӵİ뾶Ϊrcm�������ⳤΪ��
=4��ͭԭ�ӵİ뾶Ϊrcm�������ⳤΪ��![]() 4rcm=
4rcm= ![]() rcm������
rcm������![]()
![]() ��ã�r=
��ã�r=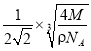
����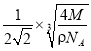 cm
cm

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��ijѧ��Ϊ̽��п�����ᷴӦ�����е����ʱ仯����![]() ʱ����100 mL 2 mol��L-1�����м��������п�ۣ�����������(�ѻ���ɱ�״��)�ۼ�ֵ���£�
ʱ����100 mL 2 mol��L-1�����м��������п�ۣ�����������(�ѻ���ɱ�״��)�ۼ�ֵ���£�
ʱ��(min) | 1 | 2 | 3 | 4 | 5 |
�������(mL) | 50 | 120 | 232 | 290 | 310 |
(1)����2~3 minʱ����ڣ��������Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ_______.
����0~5min�ڣ���Ӧ��������ʱ�����___(�� ��1~2 min������2~3 min������3~4 min��)��
(2)����ȫ��Ӧ��ų�15.2 kJ����������ӦZn(s) + 2HCl(aq)=ZnCl2(aq) + H2(g)����H=____
(3)Ϊ�˼�����Ӧ���ʵ������ٲ����������������Ӧ���зֱ��������������Һ�壬����Ϊ���е���_______(����ĸ)��
a. ����ˮ b. Na2CO3��Һ c. NaNO3��Һ
(4)Ϊ�˼ӿ췴Ӧ���ʵ������ٲ������������ijͬѧ��Ӧ���м���������CuSO4���壬��ͬѧ����____(�� ��������������������)��������_______
����Ŀ�����ݱ�Ţ�~�������Ԫ�������ڱ��е�λ�ã��ش��������⣺
�� | �� | �� | �� | �� | �� | �� | 0 | |
1 | �� | �� | ||||||
2 | �� | �� | �� | �� | ||||
3 | �� | �� | �� | �� |
(1)����+1�ۣ�������-1�۵�Ԫ����____(��Ԫ�ط���):�ڵķ���ʽ��____
(2)����߰�ԭ�Ӹ�����1:1�γɻ�����ף������ʽΪ____������еμ�����ˮʱ������Ӧ�Ļ�ѧ����ʽ��____
(3)�ߡ��ࡢ������Ԫ������������Ӧ��ˮ���������ǿ������˳������Ϊ____(�ѧʽ);�������������Ӧ��ˮ����Ļ�ѧʽΪ____
(4)�١��ݡ�������Ԫ���γɵ�һ�ֳ����εĻ�ѧʽΪ____/span>�����к��еĻ�ѧ��Ϊ____
(5)���������ɢ�����γɵĻ�����ʱ�������____ɫ��������ں��պ���Ͻ���ϵ��Ʊ�����ҵ��ұ���õ��ʵĻ�ѧ����ʽΪ____
(6)Ԫ�آ�ĵ��ʺ͢ߵ�����������ˮ����֮�䷢����Ӧ�����ӷ���ʽΪ____