��Ŀ����
��1����֪��N2(g)+O2(g)=2NO(g)����H= +180.5kJ/mol
4NH3(g)+5O2(g)=4NO(g)+6H2O(g)����H=��905kJ/mol
2H2(g)+O2(g)=2H2O(g)����H=��483.6kJ/mol
��N2(g)+3H2(g)=2NH3(g)�ġ�H= ��
��2����ҵ�ϳɰ��ķ�ӦΪN2(g)+3H2(g) 2NH3(g)����һ���¶��£���һ������N2��H2ͨ��̶����Ϊ1L���ܱ������дﵽƽ��ı�������������ʹƽ��������Ӧ�����ƶ����� ��
2NH3(g)����һ���¶��£���һ������N2��H2ͨ��̶����Ϊ1L���ܱ������дﵽƽ��ı�������������ʹƽ��������Ӧ�����ƶ����� ��
������ѹǿ ��ͨ��He
��ʹ�ô��� �ܽ����¶�
��3����ҵ�ϳɰ��ķ�ӦΪN2(g)+3H2(g) 2NH3(g)�������ݻ�Ϊ2.0L���ܱ������г���0.60molN2(g)��1.60molH2(g)����Ӧ��һ�������´ﵽƽ��ʱ��NH3�����ʵ���������NH3�����ʵ����뷴Ӧ��ϵ���ܵ����ʵ���֮�ȣ�Ϊ
2NH3(g)�������ݻ�Ϊ2.0L���ܱ������г���0.60molN2(g)��1.60molH2(g)����Ӧ��һ�������´ﵽƽ��ʱ��NH3�����ʵ���������NH3�����ʵ����뷴Ӧ��ϵ���ܵ����ʵ���֮�ȣ�Ϊ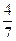 ������������´ﵽƽ��ʱN2ת����Ϊ ��
������������´ﵽƽ��ʱN2ת����Ϊ ��
��4���ϳɰ���ԭ��������һ�����͵���ɫ��Դ�����й����ķ�չǰ������������ȼ�ϵ�ؽ�����ͼ��ʾʵ�飺������c��d��Ϊ̼����NaCl��Һ�����Ϊ500ml��
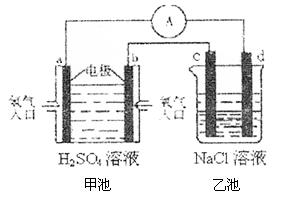
��b��Ϊ �����缫��Ӧʽ ��
c��Ϊ �����缫��Ӧʽ
����ͼװ���У���b�������ĵ�O2�ڱ�״���µ����Ϊ280mlʱ�����ҳ���Һ��PHΪ �����跴Ӧǰ����Һ������䣬��NaCl��Һ������
4NH3(g)+5O2(g)=4NO(g)+6H2O(g)����H=��905kJ/mol
2H2(g)+O2(g)=2H2O(g)����H=��483.6kJ/mol
��N2(g)+3H2(g)=2NH3(g)�ġ�H= ��
��2����ҵ�ϳɰ��ķ�ӦΪN2(g)+3H2(g)
 2NH3(g)����һ���¶��£���һ������N2��H2ͨ��̶����Ϊ1L���ܱ������дﵽƽ��ı�������������ʹƽ��������Ӧ�����ƶ����� ��
2NH3(g)����һ���¶��£���һ������N2��H2ͨ��̶����Ϊ1L���ܱ������дﵽƽ��ı�������������ʹƽ��������Ӧ�����ƶ����� ��������ѹǿ ��ͨ��He
��ʹ�ô��� �ܽ����¶�
��3����ҵ�ϳɰ��ķ�ӦΪN2(g)+3H2(g)
 2NH3(g)�������ݻ�Ϊ2.0L���ܱ������г���0.60molN2(g)��1.60molH2(g)����Ӧ��һ�������´ﵽƽ��ʱ��NH3�����ʵ���������NH3�����ʵ����뷴Ӧ��ϵ���ܵ����ʵ���֮�ȣ�Ϊ
2NH3(g)�������ݻ�Ϊ2.0L���ܱ������г���0.60molN2(g)��1.60molH2(g)����Ӧ��һ�������´ﵽƽ��ʱ��NH3�����ʵ���������NH3�����ʵ����뷴Ӧ��ϵ���ܵ����ʵ���֮�ȣ�Ϊ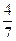 ������������´ﵽƽ��ʱN2ת����Ϊ ��
������������´ﵽƽ��ʱN2ת����Ϊ ����4���ϳɰ���ԭ��������һ�����͵���ɫ��Դ�����й����ķ�չǰ������������ȼ�ϵ�ؽ�����ͼ��ʾʵ�飺������c��d��Ϊ̼����NaCl��Һ�����Ϊ500ml��

��b��Ϊ �����缫��Ӧʽ ��
c��Ϊ �����缫��Ӧʽ

����ͼװ���У���b�������ĵ�O2�ڱ�״���µ����Ϊ280mlʱ�����ҳ���Һ��PHΪ �����跴Ӧǰ����Һ������䣬��NaCl��Һ������
��1����H=��92.4kJ/mol ��2�֣�
��2���� �� ��2�֣�
��3��66.67% ��2/3 ��2�֣�
��4����������O2+4H++4e-=2H2O��������2Cl--2e-=Cl2����ÿ��2�֣���8�֣�
�� 13��2�֣�
��2���� �� ��2�֣�
��3��66.67% ��2/3 ��2�֣�
��4����������O2+4H++4e-=2H2O��������2Cl--2e-=Cl2����ÿ��2�֣���8�֣�
�� 13��2�֣�
��

��ϰ��ϵ�д�
�����Ŀ
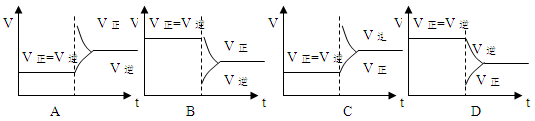

 CH3OH(g)����10 min��Ӧ�ﵽƽ��ʱ��ø���ֵ�Ũ�����£�
CH3OH(g)����10 min��Ӧ�ﵽƽ��ʱ��ø���ֵ�Ũ�����£� 4NO(g)+6H2O (g)+1025 kJ
4NO(g)+6H2O (g)+1025 kJ 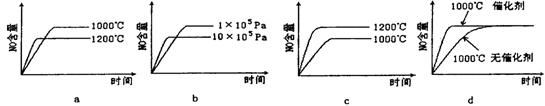
 p C��ij�¶��´ﵽƽ�⡣
p C��ij�¶��´ﵽƽ�⡣ 2NO2������������˵���Ѵﵽƽ��״̬����
2NO2������������˵���Ѵﵽƽ��״̬����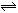 2C��������2min��Ӧ�ﵽƽ��ʱ����û�����干3.4 mol������0.4 mol C�������м�������ȷ����
2C��������2min��Ӧ�ﵽƽ��ʱ����û�����干3.4 mol������0.4 mol C�������м�������ȷ����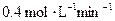
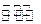 2TaS2��s��+2I2��g����t min��ﵽƽ�⣬��ʱ����2a mol I2������˵����ȷ���� �� ��
2TaS2��s��+2I2��g����t min��ﵽƽ�⣬��ʱ����2a mol I2������˵����ȷ���� �� ��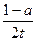 mol����L��min��
mol����L��min�� 2C(g)����H��0
2C(g)����H��0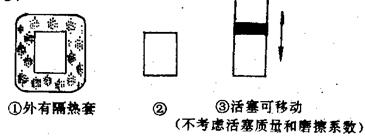
 �����о�Ͷ��3 mol A��1 mol B����ﵽƽ��ʱ����������C���ʵ����ʵ���Ũ�Ȣ� �ۣ���<��>��=����
�����о�Ͷ��3 mol A��1 mol B����ﵽƽ��ʱ����������C���ʵ����ʵ���Ũ�Ȣ� �ۣ���<��>��=����