��Ŀ����
14��������̼�������ﶼ���������⣬Խ��Խ�������ǵ����ӣ���1�����й��ڵ�����̼������������У���ȷ���Ǣ٢ۢݣ�
�ٵ������������γ��������Ҫԭ��֮һ
��SO2����Ư�����ã�������Ѭ���ʽ������������ʡ�����������
��NO������Ѫ�ܡ����ߡ���ǿ����ȷ�������Ҫ����
��NO2��CO���ܸ�Ѫ�쵰��϶���������ȱ���ж�
�ݵ���������̼�⻯���ᆳ���������䷢����Ӧ�γɹ⻯ѧ������
��2�����������������ϰ�װ����ת���������ò����ٺϽ���������������ʹCO��NO��Ӧ���ɿɲ��������̬����ѭ���������壬д��CO��NO��Ӧ�Ļ�ѧ����ʽ2NO+2CO$\frac{\underline{\;����\;}}{\;}$ N2+2CO2�������ﳣ����NaOH��Һ����NO��NO2�Ļ�����壬ʹ��ת��Ϊ������ƷNaNO2����д���仯ѧ��Ӧ����ʽNO+NO2+NaOH�TNaNO2+H2O��
��3������Ϊ�������������;���ɲ��õĴ�ʩ��C������ţ���
������ú��ȼ�ϡ� �ڰѹ����̴���� ��ȼ������ �������ữ�������м�ʯ�ҡ� �ݿ�������Դ
A���٢ڢ�B���ڢۢܢ�C���٢ۢ�D���٢ۢܢ�
��4�����᳧�����������ռ�������SO2���仯ѧ��Ӧ����ʽΪ2NaOH+SO2=Na2SO3+H2O��
���� ��1���������γ���Ҫ�������ͺ����������ꣻ
�ڶ����������ж����壻
��NO������Ѫ�ܡ����ߡ���ǿ����ȷ�������Ҫ���ã�
��NO��CO���Ѫ�쵰��϶���������ȱ���ж���
�ݴ����е�̼�⻯�����NOx��Ϊһ����Ⱦ���̫�������������������ܷ�����ѧ��Ӧ���������ֶ�����Ⱦ���һ����Ⱦ��Ͷ�����Ⱦ��Ļ�������Ϳ�������γɵ�������Ⱦ����Ϊ�⻯ѧ������
��2��CO��NO��Ӧ������������Ϊ�����Ͷ�����̼��NaOH��Һ����NO��NO2�Ļ�����壬ʹ��ת��Ϊ������ƷNaNO2��ˮ��
��3������ú��ȼ�ϡ�ȼ���������µ���Դ�ȴ�ʩ���Լ��ٶ�������������ŷţ��Ӷ�����������γɣ��ѹ����̴���ߡ������ữ�������м�ʯ�ҵȴ�ʩ������Ч�ط�ֹ������γɣ�
��4���������������������Һ��Ӧ�����������ƺ�ˮ��
��� �⣺��1���������γ���Ҫ�������ͺ����������꣬�������������γ��������Ҫԭ��֮һ���ʢ���ȷ��
��SO2����Ư�����ã����ж���������Ѭ���ʽ������������ʡ����������ã��ʢڴ���
��NO������Ѫ�ܡ����ߡ���ǿ����ȷ�������Ҫ���ã��ʢ���ȷ��
��NO��CO���Ѫ�쵰��϶���������ȱ���ж���NO2�����ܸ�Ѫ�쵰��ϣ��ʢܴ���
�ݵ�����������̼�⻯���ᆳ����������ɷ�����Ӧ�γ��ж���������Ϊ�⻯ѧ�������ʢ���ȷ��
��ѡ�٢ۢݣ�
��2������Ϣ��֪������ת������ʹCO��NO��Ӧ������������Ϊ�����Ͷ�����̼���÷�ӦΪ2NO+2CO$\frac{\underline{\;����\;}}{\;}$N2+2CO2�������ﳣ����NaOH��Һ����NO��NO2�Ļ�����壬ʹ��ת��Ϊ������ƷNaNO2����Ӧ�Ļ�ѧ����ʽΪ��NO+NO2+NaOH�TNaNO2+H2O��
�ʴ�Ϊ��2CO+2NO$\frac{\underline{\;����\;}}{\;}$N2+2CO2��NO+NO2+NaOH�TNaNO2+H2O��
��3������ú��ȼ�ϡ�ȼ���������µ���Դ�ȴ�ʩ���Լ��ٶ�������������ŷţ��Ӷ�����������γɣ�
��ѡ��C��
��4�����᳧�����������ռ�������SO2���������������������������������Һ��Ӧ�����������ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ��2NaOH+SO2=Na2SO3+H2O��
�ʴ�Ϊ��2NaOH+SO2=Na2SO3+H2O��
���� ���⿼���˵����������ʼ���Ӧ�÷����������������ʺͷ�Ӧ�����ж��ǽ���ؼ�����Ŀ�ϼ�

CH4��g��+CO2��g��=2CO��g��+2H2��g����H1
CH4��g��+H2O��l��=CO��g��+3H2��g����H2
������$\frac{��{H}_{1}}{��{H}_{2}}$���ڣ�������
| A�� | $\frac{2b-2c-a}{3b+c+a}$ | B�� | $\frac{2b+2c-a}{3b-c-a}$ | C�� | $\frac{2b+c-2a}{3b+2c-a}$ | D�� | $\frac{2b+2c-a}{3b+c-a}$ |
| A�� | �����ʵ�����Ba ��OH��2��NH4HSO4����Һ�з�Ӧ��Ba2++2OH-+NH4++H++SO42-��BaSO4��+NH3•H2O+H2O | |
| B�� | ������������������������Һ��Ӧ��Fe��OH��3+3H+��Fe3++3H2O | |
| C�� | ���廯������Һ��ͨ������������2Fe2++Cl2��2Fe3++2Cl- | |
| D�� | ����������KIO3��Һ��KI��Һ��Ӧ����I2��IO3-+5I-+3H2O��3I2+6OH- |
| A�� | �л��ﶼ�Ǵ��л����з����ȥ������ | |
| B�� | �л��ﳣ���¶��ǹ��� | |
| C�� | �л��ﲻһ����������ˮ | |
| D�� | �����л��ﶼ���߱���������� |
| A�� | �����¶����������������� | |
| B�� | Ũ������뱣������ɫ�Լ�ƿ�У�Ũ��������Ҫ | |
| C�� | �����¶�����ͭ�Ͽ췴Ӧ | |
| D�� | ¶���ڿ����У���������Һ��Ũ�ȶ����� |
| A�� | �������ڵ�ѹǿ���ٱ仯�������жϷ�Ӧ�Ѿ��ﵽƽ�� | |
| B�� | ��������ͨ��ϡ������He������ѹǿ�������Է�Ӧ�������� | |
| C�� | �������м�������A����Ӧ�������� | |
| D�� | ����Ӧ��ƽ��ʱ�������淴Ӧ���ڽ��� |
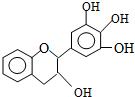 ���豶���������к��в��ӣ���������Ŀǰ�в����˹��ϳɵĴ���Ȼ����ܡ���Ч�ܵĿ������������ɻ�������������ûʳ�Ӷ����أ�EGC���Ľṹ��ͼ��ʾ������EGC��������������ȷ���ǣ�������
���豶���������к��в��ӣ���������Ŀǰ�в����˹��ϳɵĴ���Ȼ����ܡ���Ч�ܵĿ������������ɻ�������������ûʳ�Ӷ����أ�EGC���Ľṹ��ͼ��ʾ������EGC��������������ȷ���ǣ�������| A�� | ûʳ�Ӷ����أ�EGC���ܷ���ˮ�ⷴӦ | |
| B�� | 1molEGC��4molNaOHǡ����ȫ��Ӧ | |
| C�� | �ɷ���������Ӧ��ȡ����Ӧ�����ܷ����ӳɷ�Ӧ | |
| D�� | ��FeCl3��Һ�ᷢ����ɫ��Ӧ |
| A�� | ��������ˮ��Cl2+H2O�T2H++Cl-+ClO- | |
| B�� | ��ϡHNO3�еμ�Na2SO3��Һ��SO32-+2H+�TSO2��+H2O | |
| C�� | ��Al2��SO4��3��Һ�м��������NH3•H2O��Al3++4 NH3•H2O�TAlO2-+4NH4++2H2O | |
| D�� | NaHCO3��Һ�м�����Ba��OH��2��Һ��HCO3-+Ba2++OH-�TBaCO3��+H2O |
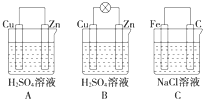 �۲�ͼA��B��C���ش��������⣺
�۲�ͼA��B��C���ش��������⣺