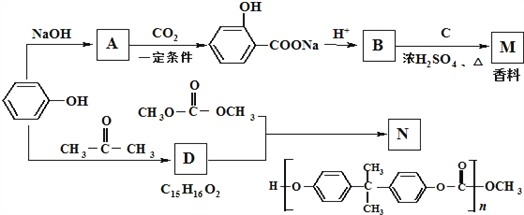
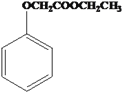
��Ŀ����
����Ŀ��ijʵ��С��������װ�ý����Ҵ���������ʵ�顣
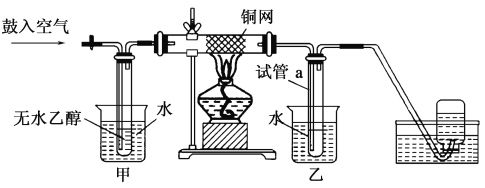
(1)ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ�����������д����Ӧ�Ļ�ѧ��Ӧ����ʽ��__________________��
�ڲ��Ϲ������������£�Ϩ��ƾ��ƣ���Ӧ���ܼ������У�˵�����Ҵ���������Ӧ��________��Ӧ��
(2)��������ˮԡ���ò���ͬ��
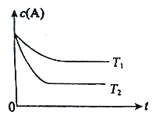
��������________���ҵ�������________��
(3)��Ӧ����һ��ʱ������Թ�a�����ռ�����ͬ�����ʣ�������________������ƿ���ռ������������Ҫ�ɷ���________��
(4)���Թ�a���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л�����________��Ҫ��ȥ�����ʣ������ڻ��Һ�м���________(��д��ĸ)��
a���Ȼ�����Һ b����
c��̼��������Һ d�����Ȼ�̼
Ȼ����ͨ��________(��ʵ���������)���ɳ�ȥ��
���𰸡�(1)2Cu��O2![]() 2CuO��CH3CH2OH��CuO
2CuO��CH3CH2OH��CuO![]() CH3CHO��Cu��H2O ����
CH3CHO��Cu��H2O ����
(2)���� ��ȴ (3)��ȩ���Ҵ���ˮ ����
(4)���� c ����
��������(1)��ʵ��ķ�Ӧԭ��Ϊ��2Cu��O2![]() 2CuO��CH3CH2OH��CuO
2CuO��CH3CH2OH��CuO![]() CH3CHO��H2O��Cu��
CH3CHO��H2O��Cu��
(2)(3)ʵ��װ���У����е�ˮΪ��ˮ���������ǽ���ˮ�Ҵ����ȳ��Ҵ������������һ����벣�����У���ͭ���������½��з�Ӧ�Ӳ������г�����������δ��Ӧ���Ҵ�����������O2��ˮ��������ȩ�����ʹ�����N2������һ��������У����е�ˮΪ��ˮ���������ǽ��Ҵ�������ˮ��������ȩ����������ȴ��ΪҺ�壬������ȴ������O2�ʹ�����N2���뵽����ƿ�С�
(4)������֪�������ʳ����ԣ�˵��������Ϊ���ᡣ��Ҫ��ȥ�Ҵ���ˮ����ȩ�е����ᣬ�����ڻ��Һ�м���NaHCO3��Һ������CH3COOH��NaHCO3��CH3COONa��H2O��CO2����Ӧ��ʹCH3COOHת��ΪCH3COONa����ͨ�����ɳ�ȥ��