��Ŀ����
NA��ʾ�����ӵ�������ֵ�������й�˵����ȷ����
A. 18.4 mol/L��Ũ�������������ˮ���������Һ�����ʵ���Ũ�ȴ���9.2 mol/L
B. �����£�1LpH��13��NaOH��Һ�У���ˮ�����OH��������ĿΪ0.1NA
C. 50 mL 14.0 mol��L-1Ũ����������ͭ��Ӧ��ת�Ƶĵ�����Ϊ0.35 NA
D. ͬ��ͬѹͬ����µ���������12C18O��14N2���еĵ��������
��ϰ��ϵ�д�
�����Ŀ
X��Y��Z��W��Ԫ�����ڱ���ԭ������������������ֶ�����Ԫ�أ��������Ϣ���±���
Ԫ�� | �����Ϣ |
X | X������������Ӧ��ˮ���ﻯѧʽΪH2XO3 |
Y | Y�ǵؿ��к�����ߵ�Ԫ�� |
Z | ZԪ�ص�ԭ���������3������ |
W | W��һ�ֺ��ص�������Ϊ28��������Ϊ14 |
������ж���ȷ����
A. Wԭ�Ӱ뾶����Z
B. ��Ԫ�ؿ�����X��Y�ֱ��γɵĶ�Ԫ��������Ӽ������γ����
C. ��Z���������ᷴӦ�����ɫ��Һ�еμ�NaOH��Һֱ������������
D. W�ĵ���������ᷴӦ����������ɫ����




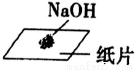


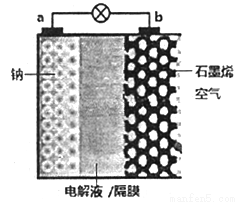
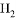 ��
�� ��0.1mol����̬��������10L���ܱ������У���ַ�Ӧ�������ⶨ
��0.1mol����̬��������10L���ܱ������У���ַ�Ӧ�������ⶨ �ı仯��5Sʱ�ⶨ
�ı仯��5Sʱ�ⶨ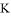 = ��
= ��