��Ŀ����
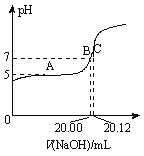
����Ŀ��ij�¶�ʱ����һ���ݻ�Ϊ2L���ܱ������У�X��Y��Z���������ʵ����ʵ�����ʱ��ı仯������ͼ��ʾ������ͼ�����ݣ�����д���пհף�
��1���÷�Ӧ�Ļ�ѧ����ʽΪ_______________��
��2����Ӧ��ʼ��2min������Z�ķ�Ӧ����Ϊ_____________��
��3����X��Y��Z��Ϊ���壬��Ӧ��ƽ��ʱ����ѹǿ�ǿ�ʼʱ��_______����������ʱ�������������СΪԭ����0.5������ƽ��ʱ���������¶Ƚ����ͣ����������������Ƚ���������÷�Ӧ������ӦΪ_________��Ӧ������ȡ������ȡ�����
���𰸡�3X+Y![]() 2Z0.05mol��L-1��min-10.9����
2Z0.05mol��L-1��min-10.9����
��������
��1����n��X��=0.3mol����n��Y��=0.1mol����n��Z��=0.2mol��������������֮��Ϊ3��1��2���ʴ�Ϊ��3X+Y![]() 2Z��
2Z��
��2��v(Z)��![]() ��
��![]() ��
��![]() =0.05molL-1min-1���ʴ�Ϊ��0.05molL-1min-1��
=0.05molL-1min-1���ʴ�Ϊ��0.05molL-1min-1��
��3��������ͼ���п�֪����ʼʱ���������ʵ���Ϊ��2mol��ƽ������������ʵ���Ϊ��0.9mol+0.7mol+0.2mol=1.8mol��![]() ��
��![]() ��
��![]() ��0.9���ʴ�Ϊ��0.9��
��0.9���ʴ�Ϊ��0.9��
��ѹǿ���ƽ�������ƶ�������ϵ�¶Ƚ��ͣ�˵������Ӧ����Ϊ���ȣ��ʴ�Ϊ�����ȡ�

����Ŀ����ͬ�¶��£��ݻ���ͬ��3�������ܱ������з������淴Ӧ��
N2��g��+3H2��g��![]() 2NH3��g�� ��H=-92.6KJ��mol-1
2NH3��g�� ��H=-92.6KJ��mol-1
ʵ������ʼ��ƽ��ʱ���й��������±���
������� | ��ʼ�����ʵ����ʵ���/mol | ��ƽ��ʱ��ϵ�����ı仯 | |||
N2 | H2 | NH3 | Ar | ||
�� | 1 | 3 | 0 | 0 | �ų�������Q1 |
�� | 0.9 | 2.7 | 0.2 | 0 | �ų�������Q2 |
�� | 0.9 | 2.7 | 0.2 | 0.1 | �ų�������Q3 |
���бȽ���ȷ����
A. �ų�������С��Q1>Q2=Q3
B. �����е�ѹǿ����>��=��
C. N2��ת���ʣ���<��<��
D. ƽ��ʱNH3�������������=��<��