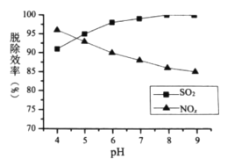
��Ŀ����
����Ŀ������±��ش���������(��Ϊ�����µ�����)��
�� | ���볣��(Ka) |
CH3COOH | 1.8��10��5 |
HClO | 3��10��8 |
H2CO3 | K1��4.4��10��7 K2��4.7��10��11 |
H2C2O4 | K1��5.4��10��2 K2��5.4��10��5 |
H2S | K1��1.3��10��7 K2��7.1��10��15 |
��ش��������⣺
(1)ͬŨ�ȵ�CH3COO����![]() ��
��![]() ��
��![]() ��ClO����S2����H����������������_____________��
��ClO����S2����H����������������_____________��
(2)������0.1molL��1��CH3COOH��Һ�ڼ�ˮϡ�����У����б���ʽ������һ����С����_____________(����ĸ)��
A��c(H��) B��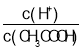 C��
C��![]() D��c(OH��)
D��c(OH��)
(3)pH��ͬ��NaClO��CH3COOK��Һ������Һ�����ʵ���Ũ�ȵĴ�С��ϵ��CH3COOK_____________NaClO������Һ�У�c(Na��)��c(ClO)_____________c(K��)��c(CH3COO��)(����������������������)��
(4)��0.1molL��1CH3COOH��Һ�еμ�NaOH��Һ��c(CH3COOH)��c(CH3COO��)��5��9����ʱ��ҺpH��_____________��
���𰸡�![]() AC �� = 5
AC �� = 5
��������
������Ӷ�Ӧ�����ĵ���ƽ�ⳣ��Խ������������������Խ����CH3COOH��Һ��ˮϡ���̣��ٽ����룬c(H+����С��c(OH-������Kw���䣻�������Խ������Ӧ��������ӵ�ˮ��̶�Խ���ж�CH3COO-��ClO-ˮ��̶ȴ�С�����õ���غ�ó�����Ũ�ȴ�С�Ĺ�ϵ����Ũ����ͬ��NaClO��CH3COOK��Һ��pH��С��ϵ�����գ�������ҺŨ�ȡ��ٽ�ˮ��������У�������ǿ��pH������CH3COOH��Һ��k=![]() ��������Һ��c(H+�������pH=-lgc(H+�����㡣
��������Һ��c(H+�������pH=-lgc(H+�����㡣
(1)ƽ�ⳣ��Խ������������������Խ�������ڵ���ƽ�ⳣ��H2C2O4��HC2O4��CH3COOH��H2CO3��H2S��HClO��HCO3��HS����ͬŨ�ȵ�CH3COO��HCO3��CO32��HC2O4��ClO��S2���H+������������ǿ��˳��Ϊ��HC2O4��CH3COO��HCO3��ClO��CO32��S2�����H+������������HC2O4��
(2)A��CH3COOH��Һ��ˮϡ���̣��ٽ����룬c(H+)��С����A��ȷ��
B��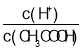 =
=![]() ��ϡ���̴ٽ�����ĵ��룬�����ӵ����ʵ�������������ʵ�����С�����Ա�ֵ���B����
��ϡ���̴ٽ�����ĵ��룬�����ӵ����ʵ�������������ʵ�����С�����Ա�ֵ���B����
C��ϡ���̣��ٽ����룬c(H+)��С��c(OH)����![]() ��С����C��ȷ��
��С����C��ȷ��
D��ϡ���̣��ٽ����룬c(H+)��С��c(OH)����D����
��ѡAC��
(3)�ݵ���ƽ�ⳣ����֪������CH3COOH��HClO����NaClO��ˮ��̶ȴ���CH3COOK��Ũ����ͬ��CH3COOK��NaClO��Һ��NaClO��ˮ��̶ȴ���CH3COOK��NaClO��Һ��pH������pH��ͬ��NaClO��CH3COOK��Һ�У�CH3COOK��Ũ�ȴ��ڴ������Һ�У����ڵ���غ㣺c(K��)+c(H��)=c(CH3COO��)+c(OH-)����c(H��)=c(CH3COO��)+c(OH-)-c(K��)����������Һ��Ҳ���ڵ���غ㣺c(Na��)+c(H��)= c(ClO-)+c(OH-)����c(H��)= c(ClO-)+c(OH-)-c(Na��)����-�ڵõ���pH��ͬ��c(H��)��ȣ�c(OH-)Ҳ��ȣ�c(Na��)��c(ClO-)=c(K��)��c(CH3COO��)��
(4)���ݴ���ĵ���ȱ���ʽCH3COOH��Һ��k=![]() =1.8��105��c(CH3COOH)��c(CH3COO)=5��9���õ���Һ��c(H+)=1.8��105��
=1.8��105��c(CH3COOH)��c(CH3COO)=5��9���õ���Һ��c(H+)=1.8��105��![]() =105mol/L������pH=5��
=105mol/L������pH=5��