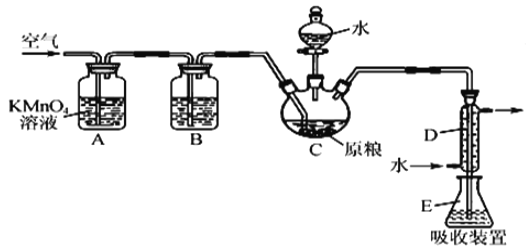
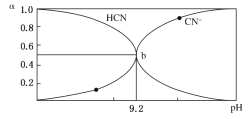
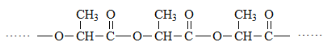��Ŀ����
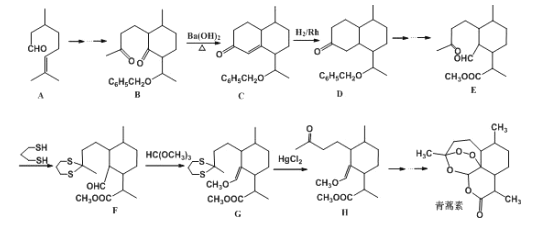
����Ŀ���ҹ�Ůҩѧ���������������Ϳ�űҩ�����غ�˫�������ض����2015��ŵ��������ѧ��ҽѧ���������ص�һ�ֻ�ѧ�ϳɷ����IJ��ֹ���������ͼ��ʾ��
��֪����C6H5-��ʾ��������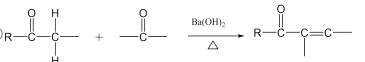
��1��������E�к��еĺ�����������__��__���ʻ���д���ƣ���
��2���ϳ�·�������E��F��G��H��Ŀ����__��
��3��B��Cʵ�����Ƿ�������Ӧ���еģ��Ƚ��мӳɷ�Ӧ���ٽ���__��Ӧ��
��4��A��Sn-p��ʯ�����£�������ͬ���칹�������մ�����֪�����մ���������3������̼ԭ�ӣ������ĸ���ͬ���ŵ�̼ԭ�ӳ�Ϊ����̼ԭ�ӣ��������մ���������ˮ������H21:1����1��4-�ӳɿ�����![]() ���������մ��Ľṹ��ʽΪ��__��
���������մ��Ľṹ��ʽΪ��__��
��5��A��ͬ���칹��Y����ȩ������Ԫ̼�����һ���ֻ��һ��֧������������������Y��__�֣����к˴Ź������������ٵ����ʵĽṹ��ʽΪ__��
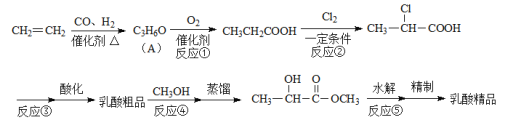
��6����ͼ������ϩΪԭ���Ʊ��л���ȩ![]() �ĺϳ�·������ͼ��
�ĺϳ�·������ͼ��
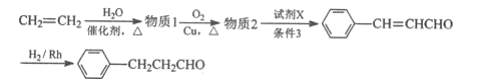
����д���пհף��л���д�ṹ��ʽ����
����1Ϊ__������2Ϊ__���Լ�XΪ__������3Ϊ__��
���𰸡�ȩ�� ���� �����ʻ� ��ȥ��Ӧ  5
5 ![]() CH3CH2OH CH3CHO
CH3CH2OH CH3CHO ![]() ��������������
��������������
��������
(1)����E�Ľṹ��ʽ���ó����������ŵ����ƣ�
(2)�Ա����ǵĽṹ��ʽ���ᷢ�������������Ŀ���DZ����ʻ���
(3)�Ա�B��C�ṹ��ʽ��C����һ��̼̼˫��������һ���ʻ����漰�ķ�Ӧ���ͼӳɷ�Ӧ����ȥ��Ӧ��
(4)���ݼӳɺ�IJ�������մ�Ӧ������Ԫ���������մ��ܷ�����������ˮ��˵�������ǻ���Ȼ����������1��1����1��4���ӳɣ�˵����������ˮ��������̼̼˫����������̼̼˫��ͨ��һ����C��C���������Ӷ�д�������մ��Ľṹ��ʽ��
(5)����A�Ľṹ��ʽ���ó�A�ķ���ʽΪC10H18O��ͬ���칹���к���ȩ������Ԫ̼������ʣ������̼ԭ���Ե�������ʽ������Ȼ���ж�ͬ���칹�����Ŀ��
(6)����1��CH2=CH2��H2O�����ӳɷ�Ӧ�IJ����ΪCH3CH2OH��CH3CH2OH�������������õ�����2����ΪCH3CHO��������Ϣ�ڣ��Ƴ��Լ�XΪ����ȩ��
(1)���ݻ�����E�Ľṹ��ʽ��E�к������������ʻ���ȩ����������
(2)�Ա�E��F�Ľṹ��ʽ��E���ʻ�������Ӧ���Ա�G��H�Ľṹ��ʽ�����ʻ������ɣ���������������Ŀ���DZ����ʻ���
(3)�Ա�B��C�ṹ��ʽ��C������һ���ʻ�������һ��̼̼˫���������ȷ����ӳɷ�Ӧ��Ӧ����Ԫ���ϵ��ʻ���H2�����ӳɷ�Ӧ�������ǻ���Ȼ����ǻ��ٷ�����ȥ��Ӧ��
(4) ���ݼӳɺ�IJ�������մ�Ӧ������Ԫ���������մ��ܷ�����������ˮ��˵�������ǻ���Ȼ����������1��1����1��4���ӳɣ�˵����������ˮ��������̼̼˫����������̼̼˫��ͨ��һ����C��C�������������մ��к�����������̼ԭ�ӣ������մ��Ľṹ��ʽΪ ��
��
(5)����A�Ľṹ��ʽ���Ƴ�A�ķ���ʽΪC10H18O��ͬ���칹���к�����Ԫ̼����ȩ��������A�IJ����Ͷ�Ϊ2���Ƴ�ʣ������̼ԭ���Ե�����ʽ��ϣ����С�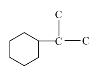 ��
��![]() ����ȩ����֧��λ����5�֣��˴Ź�����������Ӧ�ǶԳƽṹ�����ṹ��ʽΪ
����ȩ����֧��λ����5�֣��˴Ź�����������Ӧ�ǶԳƽṹ�����ṹ��ʽΪ![]() ��
��
(6)��ϩ��H2O�ڴ��������·����ӳɷ�Ӧ�������Ҵ���������1ΪCH3CH2OH��CH3CH2OH����������������ȩ��������2ΪCH3CHO��������Ϣ�ڣ��Ƴ��Լ�XΪ����ȩ����ṹ��ʽΪ![]() ��������Ϣ2�ķ�Ӧ����������3Ϊ�������������ȡ�
��������Ϣ2�ķ�Ӧ����������3Ϊ�������������ȡ�