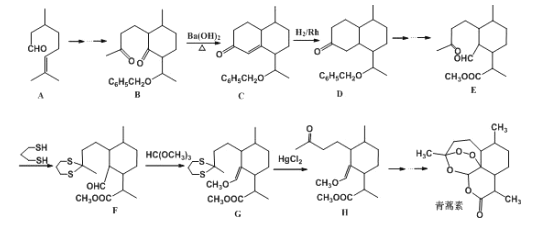
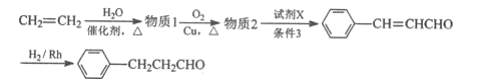
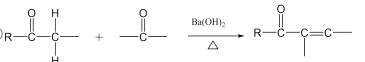��Ŀ����
����Ŀ����֪������������A��Ħ������Ϊ32g��mol��1������������Ʒ�Ӧ��E�ķ���ʽΪC3H6O2���й����ʵ�ת����ϵ��ͼ��

��ش�
(1)D�к��еĹ�����������__��
(2)д���л���E��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ__��
(3)����˵����ȷ����_��
A���л���C��ˮ��Һ�����ڱ걾�ķ���
B���л���B��D����������Ʒ�����Ӧ
C���л���E�л���D�����ñ���̼������Һ���з���
D���л���MΪ�߷��ӻ�����
���𰸡��Ȼ� CH3COOCH3+NaOH![]() CH3COONa+CH3OH BC
CH3COONa+CH3OH BC
��������
����������A��Ħ������Ϊ32g��mol��1������������Ʒ�Ӧ����A�����к���-OH���ṹ��ʽΪCH3OH��E�ķ���ʽΪC3H6O2����D�ķ����к���2��̼ԭ�ӣ�����B�������������ɣ���DΪCH3COOH��CΪCH3CHO��BΪCH3CH2OH��EΪCH3COOCH3��M�ھƻ�ø����������B����MΪ�����ǡ�
(1)�����Ϸ�����֪��DΪCH3COOH�����еĹ������������Ȼ�����Ϊ���Ȼ���
(2)�������ƶϿ�֪���л���EΪCH3COOCH3����E��NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪCH3COOCH3+NaOH![]() CH3COONa+CH3OH����Ϊ��CH3COOCH3+NaOH
CH3COONa+CH3OH������CH3COOCH3+NaOH![]() CH3COONa+CH3OH��
CH3COONa+CH3OH��
(3)A���л���CΪCH3CHO����HCHO��ˮ��Һ�����ڱ걾�ķ�����A����ȷ��
B���л���BΪCH3CH2OH��DΪCH3COOH�����Ƕ���������Ʒ�����Ӧ����H2��B��ȷ��
C���л���E(CH3COOCH3)�л���D(CH3COOH)�����ݳ�ȥ���������л��е�����������ƣ����ñ���̼������Һ���з��룬C��ȷ��
D���л���MΪ�����ǣ���Է�������Ϊ180�������ڸ߷��ӻ����D����ȷ��
��ѡBC��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�