ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΖœΥ°÷–Α±Χ§ΒΣ“‘NH3ΓΛH2OΓΔNH3ΚΆNH4+ΒΡ–Έ Ϋ¥φ‘ΎΘ§ΖœΥ°Ά―ΒΣ“―≥…ΈΣ÷ς“ΣΈέ»ΨΈοΦθ≈≈ΚΆΥ°ΧεΗΜ”Σ―χΜ·Ζά÷ΈΒΡ―–ΨΩ»»ΒψΓΘ
ΔώΘ°≥ΝΒμΖ®
œρΖœΥ°÷–ΆΕ»κMgCl2ΚΆNa2HPO4Θ§…ζ≥…MgNH4PO4ΓΛ6H2O≥ΝΒμΘ§Ω…ΫΪΑ±Χ§ΒΣΚ§ΝΩΫΒ÷Ν10mgΓΛL1“‘œ¬ΓΘ
Θ®1Θ©NH3ΒΡΒγΉ” ΫΘΚ______ΓΘ
Θ®2Θ©ΖœΥ°÷–ΒΡNH3ΓΛH2OΉΣΜ·ΈΣMgNH4PO4ΓΛ6H2OΒΡάκΉ”ΖΫ≥Χ Ϋ «______ΓΘ

Θ®3Θ©16Γφ ±Θ§œρΖœΥ°÷–Φ”»κMgCl2ΚΆNa2HPO4Θ§ ΙΟΨΓΔΒΣΓΔΝΉΈο÷ ΒΡΝΩ÷°±»ΈΣ1©U1©U1Θ§≥ΝΒμΙΐ≥Χ÷–ΒΡpHΕ‘ Θ”ύΑ±Χ§ΒΣ≈®Ε»ΒΡ”Αœλ»γΆΦΓΘ”ϊ Ι Θ”ύΑ±Χ§ΒΣ≈®Ε»ΒΆ”Ύ10mgΓΛL1Θ§pHΒΡ “ΥΖΕΈß «______Θ§pHΤΪ¥σΜρ’ΏΤΪ–ΓΨυ≤Μάϊ”ΎMgNH4PO4ΓΛ6H2OΒΡ…ζ≥…ΓΘ
ΔρΘ°ΈΔ≤®―θΜ·Ζ®
Θ®4Θ©ΈΔ≤®–≠Ά§CuOΚΆH2O2≥ΐ»ΞΑ±Χ§ΒΣ
ΔΌΤδΥϊΧθΦΰœύΆ§Θ§»ΓœύΆ§ΧεΜΐΒΡΆ§“ΜΖœΥ°―υΤΖΘ§ΈΔ≤®10 minΘ§ Θ”ύΑ±Χ§ΒΣ≈®Ε»”κ“ΜΕ®≈®Ε»H2O2»ή“ΚΧμΦ”ΝΩΒΡΙΊœΒ»γœ¬ΆΦΓΘΨίΆΦΆΤ≤βCuO‘ΎΑ±Χ§ΒΣΆ―≥ΐ÷–Ω…ΡήΤπ¥ΏΜ·Ής”ΟΘ§άμ”…_____________
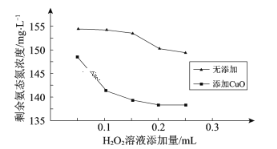
ΔΎΈΔ≤®–≠Ά§CuO”–άϊ”ΎH2O2≥ΐ»ΞΑ±Χ§ΒΣΓΘΗΟΧθΦΰœ¬Θ§H2O2ΫΪNH3―θΜ·ΈΣN2ΒΡΜ·―ßΖΫ≥Χ Ϋ «____________________ΓΘ
ΓΨ¥πΑΗΓΩ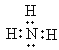 Mg2+ + NH3ΓΛH2O+HPO42 + 5H2O = MgNH4PO4ΓΛ6H2OΓΐ pHΘΫ8~10ΘΜ pHΤΪ¥σΘ§ NH4+ΓΔMg2+“Ή”κOHΫαΚœ…ζ≥…NH3ΓΛH2OΓΔMg(OH)2Θ§ NH3ΓΛH2OΒΡΒγάκ±Μ“÷÷ΤΘ§ ΙNH4+ΚΆMg2+≈®Ε»ΫΒΒΆΘΜpHΤΪ–ΓΘ§≤Μάϊ”ΎHPO42ΒγάκΘ§PO43≈®Ε»ΤΪΒΆΓΘΥυ“‘pHΤΪ¥σΜρΤΪ–ΓΨυ≤Μάϊ”ΎMgNH4PO4ΓΛ6H2OΒΡ…ζ≥…ΘΜ œύΆ§ΒΡH2O2»ή“ΚΧμΦ”ΝΩΘ§œύΆ§ ±ΦδΡΎΘ§”κ≤ΜΦ”CuOœύ±»Θ§Φ”»κCuOΘ§Α±Χ§ΒΣ≈®Ε»ΫΒΒΆΒΡΕύΘ§Ζ¥”ΠΥΌ¬ Ωλ 3H2O2+ 2NH3
Mg2+ + NH3ΓΛH2O+HPO42 + 5H2O = MgNH4PO4ΓΛ6H2OΓΐ pHΘΫ8~10ΘΜ pHΤΪ¥σΘ§ NH4+ΓΔMg2+“Ή”κOHΫαΚœ…ζ≥…NH3ΓΛH2OΓΔMg(OH)2Θ§ NH3ΓΛH2OΒΡΒγάκ±Μ“÷÷ΤΘ§ ΙNH4+ΚΆMg2+≈®Ε»ΫΒΒΆΘΜpHΤΪ–ΓΘ§≤Μάϊ”ΎHPO42ΒγάκΘ§PO43≈®Ε»ΤΪΒΆΓΘΥυ“‘pHΤΪ¥σΜρΤΪ–ΓΨυ≤Μάϊ”ΎMgNH4PO4ΓΛ6H2OΒΡ…ζ≥…ΘΜ œύΆ§ΒΡH2O2»ή“ΚΧμΦ”ΝΩΘ§œύΆ§ ±ΦδΡΎΘ§”κ≤ΜΦ”CuOœύ±»Θ§Φ”»κCuOΘ§Α±Χ§ΒΣ≈®Ε»ΫΒΒΆΒΡΕύΘ§Ζ¥”ΠΥΌ¬ Ωλ 3H2O2+ 2NH3![]() N2+6H2O
N2+6H2O
ΓΨΫβΈωΓΩ
(1)NH3ΒΡΒγΉ” ΫΈΣ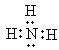 ΘΜ
ΘΜ
(2)ΗυΨίΧβ“βΘ§ΖœΥ°÷–ΒΡNH3ΓΛH2OΉΣΜ·ΈΣMgNH4PO4ΓΛ6H2OΘ§Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣMg2+ + NH3ΓΛH2O+HPO42 + 5H2O = MgNH4PO4ΓΛ6H2OΓΐΘΜ
(3)”…ΆΦΩ…“‘Ω¥≥ωΘ§”ϊ Ι Θ”ύΑ±Χ§ΒΣ≈®Ε»ΒΆ”Ύ10mgΓΛL1Θ§pH “ΥΖΕΈßΈΣ8~10Θ§pHΤΪ¥σΘ§ NH4+ΓΔMg2+“Ή”κOHΫαΚœ…ζ≥…NH3ΓΛH2OΓΔMg(OH)2Θ§ NH3ΓΛH2OΒΡΒγάκ±Μ“÷÷ΤΘ§ ΙNH4+ΚΆMg2+≈®Ε»ΫΒΒΆΘΜpHΤΪ–ΓΘ§≤Μάϊ”ΎHPO42ΒγάκΘ§PO43≈®Ε»ΤΪΒΆΓΘΥυ“‘pHΤΪ¥σΜρΤΪ–ΓΨυ≤Μάϊ”ΎMgNH4PO4ΓΛ6H2OΒΡ…ζ≥…ΘΜ
(4)ΔΌ”…ΆΦΩ…÷ΣΘ§‘ΎœύΆ§ ±ΦδΚΆH2O2»ή“ΚΧμΦ”ΝΩœύΆ§ ±Θ§ΧμΦ”ΝΥCuOΒΡΖ¥”ΠΥΌ¬ ΫœΩλΘ§Α±Χ§ΒΣΒΡ Θ”ύ≈®Ε»‘Ε‘Ε–Γ”ΎΈ¥ΧμΦ”CuOΘΜ
![]() ΔΎΗυΨί…œ ωΖ÷ΈωΘ§‘ΎΈΔ≤®–≠Ά§CuOΉς”Οœ¬Θ§H2O2ΚΆNH3…ζ≥…N2Θ§Ζ¥”ΠΒΡΖΫ≥Χ ΫΈΣ3H2O2+ 2NH3
ΔΎΗυΨί…œ ωΖ÷ΈωΘ§‘ΎΈΔ≤®–≠Ά§CuOΉς”Οœ¬Θ§H2O2ΚΆNH3…ζ≥…N2Θ§Ζ¥”ΠΒΡΖΫ≥Χ ΫΈΣ3H2O2+ 2NH3![]() N2+6H2OΓΘ
N2+6H2OΓΘ

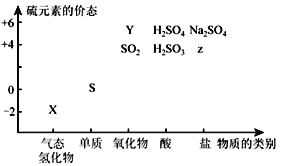
ΓΨΧβΡΩΓΩ““Ο― «”–ΜζΚœ≥…÷–≥Θ”ΟΒΡ»ήΦΝΓΘΡ≥ Β―ι–ΓΉι‘Ύ Β―ι “άϊ”Ο““¥ΦΆ―Υ°÷Τ±Η““Ο―Θ§ΉΑ÷Ο Ψ“βΆΦΘ®Φ–≥÷ΚΆΦ”»»ΉΑ÷Ο“―¬‘»ΞΘ©ΓΔ”–ΙΊ ΐΨίΚΆ Β―ι≤Ϋ÷η»γœ¬ΘΚ


Έο÷ | œύΕ‘Ζ÷Ή”÷ ΝΩ | ΟήΕ»Θ·Θ®gΓΛmLΘ≠1Θ© | Ζ–ΒψΘ·Γφ | ‘ΎΥ°÷–ΒΡ»ήΫβ–‘ |
““¥Φ | 46 | 0.816 | 78 | ΜΞ»ή |
““Ο― | 74 | 0.713 | 34.6 | ≤Μ»ή |
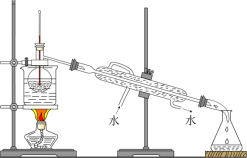
“―÷ΣΘΚΔΌœύΆ§ΧθΦΰœ¬Θ§““Ο―‘Ύ±ΞΚΆ ≥―ΈΥ°÷–±»‘ΎΥ°÷–ΗϋΡ―»ήΓΘ
ΔΎ¬»Μ·ΗΤΩ…”κ““¥Φ–Έ≥…¬γΚœΈοCaCl2ΓΛ6C2H5OHΓΘ
«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©“«ΤςBΒΡΟϊ≥ΤΈΣ________ΓΘ
Θ®2Θ©”…““¥Φ÷Τ±Η““Ο―ΒΡΉήΖ¥”ΠΈΣ2C2H5OH![]() CH3CH2OCH2CH3ΘΪH2OΘ§¥ΥΖ¥”ΠΖ÷ΝΫ≤ΫΫχ––Θ§ΒΎ“Μ≤ΫΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚCH3CH2OHΘΪH2SO4
CH3CH2OCH2CH3ΘΪH2OΘ§¥ΥΖ¥”ΠΖ÷ΝΫ≤ΫΫχ––Θ§ΒΎ“Μ≤ΫΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚCH3CH2OHΘΪH2SO4![]() CH3CH2OSO2OHΘΪH2OΘ§‘ρΒΎΕΰ≤ΫΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ_____________ΓΘ
CH3CH2OSO2OHΘΪH2OΘ§‘ρΒΎΕΰ≤ΫΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ_____________ΓΘ
Θ®3Θ©“«ΤςD÷– Δ”–±υΥ°ΜλΚœΈοΘ§ΤδΉς”ΟΈΣ____________ΓΘ
Θ®4Θ©≤ΌΉς1ΒΡΟϊ≥ΤΈΣ_______________ΓΘ
Θ®5Θ©»τ Γ¬‘≤Ϋ÷ηΔρΜαΒΦ÷¬ΒΡΚσΙϊ «________ΓΘ
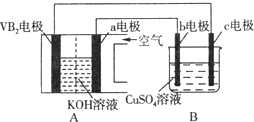
ΓΨΧβΡΩΓΩΈΣ‘ω«Ω¬ΝΒΡΡΆΗ· ¥–‘,œ÷“‘«Π–νΒγ≥ΊΈΣΆβΒγ‘¥,“‘AlΉς―τΦΪΓΔPbΉς“θΦΪ,ΒγΫβœΓΝρΥα, Ι¬Ν±μΟφΒΡ―θΜ·ΡΛ‘ωΚώΓΘΖ¥”Π‘≠άμ»γœ¬:
Βγ≥Ί:Pb(s)+PbO2(s)+2H2SO4(aq)=2PbSO4(s)+2H2O(l)
ΒγΫβ≥Ί:2Al+3H2O![]() Al2O3+3H2Γϋ
Al2O3+3H2Γϋ
ΒγΫβΙΐ≥Χ÷–,“‘œ¬≈–Εœ’ΐ»ΖΒΡ «( )
Βγ≥Ί | ΒγΫβ≥Ί | |
A | H+“ΤœρPbΒγΦΪ | H+“ΤœρPbΒγΦΪ |
B | ΟΩœϊΚΡ3molPb | …ζ≥…2molAl2O3 |
C | ’ΐΦΪ:PbO2+4H++2e-=Pb2++2H2O | ―τΦΪ:2Al+3H2O-6e-=Al2O3+6H+ |
D |
|
|
A. AB. BC. CD. D