��Ŀ����
��10�֣�25ʱ�����ȡ0.1 mol��L-1HA��Һ��0.1 mol��L-1NaOH��Һ��������(��Ϻ���Һ����ı仯����)����û����Һ��pH=8���Իش��������⣺
��1�������Һ��pH=8��ԭ�������ӷ���ʽ��ʾ����
___________________________________________________________________��
��2�������Һ����ˮ�������c(H+)_____(�������������=��)0.1 mol��L-1 NaOH��Һ����ˮ�������c(H+)��
��3��������Һ��������ʽ�ľ�ȷ��������
c (Na+)��c (A��)= ______________mol��L-1��
��4����֪NH4A��ҺΪ���ԣ���֪��HA��Һ�ӵ�Na2CO3��Һ��������ų������ƶϣ�NH4��2CO3��Һ��pH_____7 (�������������=��)��
��5������ͬ�¶�����ͬŨ�ȵ�������Һ��
�٣�NH4��2CO3 ��NH3�� H2O �ۣ�NH4��2SO4 ��NH4Cl ��CH3COONH4
��c(NH4+)�ɴ�С��˳������___________________������ţ���
��1�������Һ��pH=8��ԭ�������ӷ���ʽ��ʾ����
___________________________________________________________________��
��2�������Һ����ˮ�������c(H+)_____(�������������=��)0.1 mol��L-1 NaOH��Һ����ˮ�������c(H+)��
��3��������Һ��������ʽ�ľ�ȷ��������
c (Na+)��c (A��)= ______________mol��L-1��
��4����֪NH4A��ҺΪ���ԣ���֪��HA��Һ�ӵ�Na2CO3��Һ��������ų������ƶϣ�NH4��2CO3��Һ��pH_____7 (�������������=��)��
��5������ͬ�¶�����ͬŨ�ȵ�������Һ��
�٣�NH4��2CO3 ��NH3�� H2O �ۣ�NH4��2SO4 ��NH4Cl ��CH3COONH4
��c(NH4+)�ɴ�С��˳������___________________������ţ���
��1��A��+H2O HA+OH��
HA+OH��
��2����
��3��9.9��10��7
��4����
��5��CDBA
 HA+OH��
HA+OH�� ��2����
��3��9.9��10��7
��4����
��5��CDBA
��

��ϰ��ϵ�д�
�����Ŀ

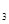 ��Һʱ��������FeCl
��Һʱ��������FeCl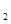 (SO
(SO )
)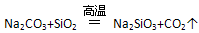 ��˵��H2SiO3����ǿ��H2CO3
��˵��H2SiO3����ǿ��H2CO3