��Ŀ����
��10�֣���������Ũ�Ⱦ�Ϊ0.05mol/L��������Һ����Na2CO3��NaHCO3��HCl ��NH3��H2O���ش�������⣺
��1��������Һ�У��ɷ���ˮ����� ������ţ�
��2��������Һ�У�������NaOH��Һ��Ӧ��������H2SO4��Һ��Ӧ����
Һ�У�����Ũ�ȴ�С�Ĺ�ϵ
��3������м�������NH4Cl���壬��ʱc(NH4+/OH-)��ֵ ������������С�����䡱 ��
��4�������ۺܵ͢���Һ��Ϻ���Һǡ�ó����ԣ�����ǰ�۵���� �ܵ�����������ڡ�����С�ڡ����ڡ� ��
��5��ȡ10mL�Ģ���Һ����ˮϡ�͵�500mL�������Һ����ˮ�������c(H+) = mol/L
��1��������Һ�У��ɷ���ˮ����� ������ţ�
��2��������Һ�У�������NaOH��Һ��Ӧ��������H2SO4��Һ��Ӧ����
Һ�У�����Ũ�ȴ�С�Ĺ�ϵ
��3������м�������NH4Cl���壬��ʱc(NH4+/OH-)��ֵ ������������С�����䡱 ��
��4�������ۺܵ͢���Һ��Ϻ���Һǡ�ó����ԣ�����ǰ�۵���� �ܵ�����������ڡ�����С�ڡ����ڡ� ��
��5��ȡ10mL�Ģ���Һ����ˮϡ�͵�500mL�������Һ����ˮ�������c(H+) = mol/L
��10�֣�
��1�� �٢�
��2�� c(Na+)�� c(HCO3��) �� c(OH��) �� c(H+)�� c(CO32��)
��3�� ���� ��4�� ��
��5�� 10-11
��1�� �٢�
��2�� c(Na+)�� c(HCO3��) �� c(OH��) �� c(H+)�� c(CO32��)
��3�� ���� ��4�� ��
��5�� 10-11
��1��������Һ�У�ֻ�к��������Ӳ���ˮ�⣬���ԣ�������Һ�пɷ���ˮ����ǣ�Na2CO3��NaHCO3��������٢�
��2��������NaOH��Һ��Ӧ��������H2SO4��Һ��Ӧ����Һָ����NaHCO3��NaHCO3��Һ�Լ��ԣ�HCO3����ˮ��̶ȴ��ڵ���̶ȡ�����Ũ�ȵĴ�С��ϵ�ǣ�c(Na+)�� c(HCO3��) �� c(OH��) �� c(H+)�� c(CO32��)
��3������м�������NH4Cl���壬�ܴ���һˮ�ϰ��ĵ���ƽ�⣬��������Ũ������ƽ�����ƣ���ʱc(NH4+/OH-)��ֵ����
��4�������ۺܵ͢���Һ��Ϻ�õ��Ȼ�泥���Һǡ�ó����ԣ���ˮӦ�ù��������ԣ����ǰ�۵����С�ڢܵ����
��5��0.05mol/L10mL��HCl��Һ����ˮϡ�͵�500mL����ʱ�����Ũ��Ϊ0.001mol/L, c(H+) = 10-3mol/L,��ôˮ�������c(H+)=10-11mol/L
��2��������NaOH��Һ��Ӧ��������H2SO4��Һ��Ӧ����Һָ����NaHCO3��NaHCO3��Һ�Լ��ԣ�HCO3����ˮ��̶ȴ��ڵ���̶ȡ�����Ũ�ȵĴ�С��ϵ�ǣ�c(Na+)�� c(HCO3��) �� c(OH��) �� c(H+)�� c(CO32��)
��3������м�������NH4Cl���壬�ܴ���һˮ�ϰ��ĵ���ƽ�⣬��������Ũ������ƽ�����ƣ���ʱc(NH4+/OH-)��ֵ����
��4�������ۺܵ͢���Һ��Ϻ�õ��Ȼ�泥���Һǡ�ó����ԣ���ˮӦ�ù��������ԣ����ǰ�۵����С�ڢܵ����
��5��0.05mol/L10mL��HCl��Һ����ˮϡ�͵�500mL����ʱ�����Ũ��Ϊ0.001mol/L, c(H+) = 10-3mol/L,��ôˮ�������c(H+)=10-11mol/L

��ϰ��ϵ�д�
�����Ŀ
 H++A2-����֪������Ũ��Ϊ0.1mol/L��H2A��Һ��c(H+)��0.11mol/L�������0.1mol/L��NaHA��Һ��������������ȷ���ǣ��� ��
H++A2-����֪������Ũ��Ϊ0.1mol/L��H2A��Һ��c(H+)��0.11mol/L�������0.1mol/L��NaHA��Һ��������������ȷ���ǣ��� ��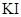 ��Ϊ0.01mol/L����Һ�м���8mL0.01mol/L��AgNO3��Һ,��ʱ��Һ���������ʵ�����Ũ�ȴ�С��ϵ��ȷ����
��Ϊ0.01mol/L����Һ�м���8mL0.01mol/L��AgNO3��Һ,��ʱ��Һ���������ʵ�����Ũ�ȴ�С��ϵ��ȷ����  mol/L��Na2CO3��FeCl3��Һ����Na2CO3��Һ�е����̪������Һ����________(�dz�족��ɫ����ԭ��__________________________(�����ӷ��̱���)�����ȣ�����ɫ��_________(dz����)����FeCl3��Һ�е���ʯ�������Һ����________������ɫ�������������ɲ����գ���õ��Ĺ�������Ϊ��__________________________���������͵�FeCl3�����ˮ�������ķ�Ӧ�� _________________________(�����ӷ��̱���)
mol/L��Na2CO3��FeCl3��Һ����Na2CO3��Һ�е����̪������Һ����________(�dz�족��ɫ����ԭ��__________________________(�����ӷ��̱���)�����ȣ�����ɫ��_________(dz����)����FeCl3��Һ�е���ʯ�������Һ����________������ɫ�������������ɲ����գ���õ��Ĺ�������Ϊ��__________________________���������͵�FeCl3�����ˮ�������ķ�Ӧ�� _________________________(�����ӷ��̱���)