��Ŀ����
7����10mol/L��ŨH2SO4����250ml0.5mol/L��ϡH2SO4���밴Ҫ����գ�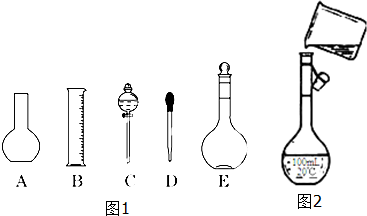
��1������ŨH2SO4�����Ϊ12.5mL�����ʵ������10mL��20mL��50mL��Ͳ��Ӧѡ��20mL��Ͳ��
��2����ͼ1��ʾ��������������Һ�϶�����Ҫ����AC��˫ѡ������ţ�������������Һ�����õ��IJ����������ձ���������������һ�֣���
��3����ͼ2�����ƹ�����ת����Һ��ʾ��ͼ��ͼ�����������ֱ��ǣ�
������ƿ�Ĺ��ѡ����
��δ�ò�����������
��4�����ƹ����У����в����ᵼ������ϡ����Ũ��ƫС����BD��˫ѡ��
A������ƿ������ˮϴ�Ӻ������������ˮ
B�����ù����ձ���������δϴ��
C������ʱ��������ƿ�̶���
D��ҡ�Ⱥ��ã�����Һ����ڿ̶��ߣ������μ�����ˮ���̶���
��5����250mL 0.5mol/LH2SO4��Һȡ��5mL����Һ������H+���ʵ���Ũ��Ϊ1mol/L��
���� ��1��������Һϡ�Ͷ���CŨVŨ=CϡVϡ�����㣻���ݡ����������ԭ������Ҫ��ȡ��Ũ����������ѡ����ʵ���Ͳ��
��2���������Ʋ����Ǽ��㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��������Ҫ�Ͳ���Ҫ��������
��3������ƿֻ��һ���̶��ߣ���ֻ�����ƺ��������Ӧ���������Һ����Һʱһ��Ҫ�ò�����������
��4������c=$\frac{n}{V}$��������ʵ����ʵ���n����Һ�����V�ı仯��������������
��5����Һ�Ǿ�һ�ȶ��ģ���Ũ������ȡ��������أ�
��� �⣺��1��������Ũ��������ΪVmL��������Һϡ�Ͷ���CŨVŨ=CϡVϡ��֪��10mol/L��VmL=250ml��0.5mol/L
���V=12.5mL��
���ݡ����������ԭ������Ҫ��ȡ��Ũ��������Ϊ12.5mL��֪Ӧѡ��20mL����Ͳ��
�ʴ�Ϊ��12.5��20��
��2���������Ʋ����Ǽ��㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��֪����������У���Ͳ���ձ�����������250mL����ƿ����ͷ�ιܣ��ʲ���Ҫ��������AC������Ҫ���������ձ��Ͳ��������ʴ�Ϊ��AC���ձ�������������
��3������ƿֻ��һ���̶��ߣ���ֻ�����ƺ��������Ӧ���������Һ��������250mL��ҺӦѡ��250mL����ƿ��������ƿ�Ĺ��ѡ������Һʱһ��Ҫ�ò�����������������Һ�������ʴ�Ϊ������ƿ�Ĺ��ѡ����δ�ò�����������
��4��A��������ƿδ���T����������Һ������ҺŨ����Ӱ�죬��ΪֻҪ����ʱ��ȷ������ˮ��ԭ�����еĻ��Ǻ�������ģ���Ũ����Ӱ�죬��A��ѡ��
B�����ù����ձ���������δϴ�ӣ����������ʵ���ʧ��ʹ������Һ��Ũ��ƫ�ͣ���Bѡ��
C������ʱ��������ƿ�̶��ߣ��ᵼ����Һ���ƫС����Ũ��ƫ�ߣ���C��ѡ��
D��ҡ�Ⱥ��ã�����Һ����ڿ̶����������ģ������μ�����ˮ���̶��ߣ���Ũ��ƫ�ͣ���Dѡ��
��ѡBD��
��5�����������Ƕ�Ԫǿ�ᣬ����250mL 0.5mol/LH2SO4��Һ�У������ӵ�Ũ��Ϊ1mol/L������Һ�Ǿ�һ�ȶ��ģ���Ũ������ȡ��������أ���ȡ��5mL��
��Һ��H+���ʵ���Ũ����Ϊ1mol/L��
�ʴ�Ϊ��1��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƹ����еļ���������������ڻ�������Ŀ���ѶȲ���

| A�� | V1=10V2 | B�� | V1��10V2 | C�� | V1��10V2 | D�� | V2��10V1 |
| ������ | ���� | ���� | ������ | |
| ������ | 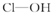 | 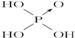 | 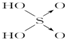 |  |
| ���ǻ���ԭ���� | 0 | 1 | 2 | 3 |
| ���� | ���� | ��ǿ�� | ǿ�� | ��ǿ�� |
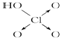
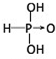 ��������
��������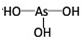 ��
����2���ֱ�д����������������������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ�������H3PO3+2NaOH=Na2HPO3+2H2O�������H3AsO3+3NaOH=Na3AsO3+3H2O��
��3���Ƚ�H3AsO4��H2CrO4��HMnO4������ǿ��������������ǿ��˳��H3AsO4��H2CrO4��HMnO4��
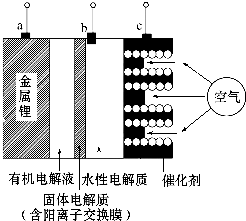
| A�� | �õ�طŵ�ʱ��ͼ�е缫a��������c�Ǹ��� | |
| B�� | �õ�طŵ�ʱ��ˮ�Ե����pH���� | |
| C�� | �������-������س��ʱ��c��Ӧ�ӵ�Դ�ĸ��� | |
| D�� | �������-������س��ʱ��b���ĵ缫��ӦʽΪ4OH--4e-=2H2O+O2�� |
��1�����д�ʩ�У������ڽ��ʹ�����CO2Ũ�ȵ���abc��������ĸ��ţ�
a�����ý��ܼ��������ٻ�ʯȼ�ϵ�����
b�������������������У�������̼����
c������̫���ܡ����ܵ�������Դ�����ʯȼ��
��2����һ��;���ǽ�CO2ת�����л���ʵ��̼ѭ�����磺
2CO2��g��+2H2O��l���TC2H4��g��+3O2��g����H=+1411.0kJ/mol
2CO2��g��+3H2O��l���TC2H5OH��1��+3O2��g����H=+1366.8kJ/mol
������ϩˮ�����Ҵ����Ȼ�ѧ����ʽ�ǣ�C2H4��g��+H2O��l��=C2H5OH��l����H=-44.2kJ/mol��
��3����һ�������£�6H2��g��+2CO2��g��?CH3CH2OH��g��+3H2O��g����
| �¶ȣ�k�� CO2ת���ʣ�%�� n��H2��/n��CO2�� | 500 | 600 | 700 | 800 |
| 1.5 | 45 | 33 | 20 | 12 |
| 2 | 60 | 43 | 28 | 15 |
| 3 | 83 | 62 | 37 | 22 |
���¶�һ��ʱ�������̼�ȣ�CO2��ת���������������С�����䡱����
�ڸ÷�Ӧ������ӦΪ�ţ�������š����ȷ�Ӧ��
����ͼһ������ϵ����ͼ��˵��ѹǿ��p1����p2ʱ������ƽ���ƶ�����H2ת���ʺ��Ҵ��ٷֺ����ı仯��

| A�� | 1��9 | B�� | 9��1 | C�� | 1��11 | D�� | 11��1 |
| A�� | ��Cl2+2KBr�T2KCl+Br2����F2Ҳ����KBr��Һ��Ӧ�û���Br2 | |
| B�� | �����£���Cu+4HNO3��Ũ���TCu��NO3��2+2NO2��+2H2O������FeҲ����Ũ���ᷴӦ����NO2 | |
| C�� | ��Cu+Cl2$\frac{\underline{\;\;��\;\;}}{\;}$CuCl2 ����Cu+I2$\frac{\underline{\;\;��\;\;}}{\;}$CuI2 | |
| D�� | ���Ʊ�����ú���У����Լ�Ҳ���Ա�����ú���� |
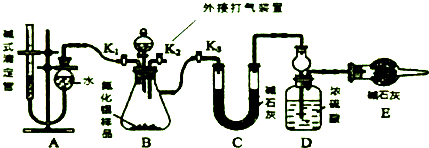
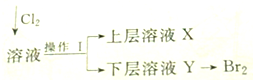 ʵ�����ú���Ca2+��Mg2+��Cl-��SO42-��Br-�����ӵ���Һ�����й�ʵ�飮��֪��Cl2+2Br-=Br2+2Cl-��
ʵ�����ú���Ca2+��Mg2+��Cl-��SO42-��Br-�����ӵ���Һ�����й�ʵ�飮��֪��Cl2+2Br-=Br2+2Cl-��