��Ŀ����
����Ŀ��ij�ϴ�����58.2%��SiO2��21.0%��ZnO��4.5%��ZnS��12.8%��CuS��ijͬѧ��15.0 g�÷ϴ���Ϊԭ�ϣ��������е�п��ͭ�����õ�ʵ�鷽�����£�
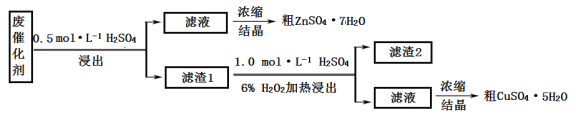
�ش��������⣺
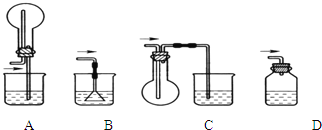
��1��������װ���У���һ�ν���������____________���ڶ��ν���Ӧѡ��____________�������ţ�
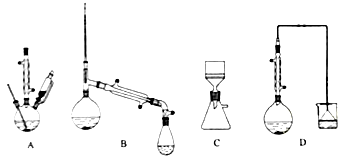
��2���ڶ��ν���ʱ����ʢ����Һ1�ķ�Ӧ���м���ϡ���ᣬ��������������Һ����˳���෴�������___________������2����Ҫ�ɷ���____________________��
��3��Ũ������п������ͭ��Һʹ�õ�����������________________��
��4��ijͬѧ��ʵ�����֮�õ�1.5gCuSO4�q5H2O,��ͭ�Ļ�����Ϊ__________________��
���𰸡�
��1��D A ��ÿ��1�֣���2�֣�
��2��H2O2���������Ӵ��ֽ⣨ÿ��2�֣���4�֣�
��3��������2�֣�
��4��30% ��3�֣�
��������
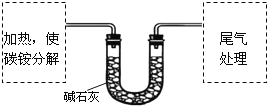
�����������1�����������ѧ��������֪��һ�ν���������ӦZnO+H2SO4![]() ZnSO4+H2O��ZnS+H2SO4
ZnSO4+H2O��ZnS+H2SO4![]() ZnSO4+H2S�����ж�����H2S���ɣ�����������������Һ����β��������ѡDװ�ã��ڶ��ν���ʱ������Ӧ��CuS+H2O2+H2SO4
ZnSO4+H2S�����ж�����H2S���ɣ�����������������Һ����β��������ѡDװ�ã��ڶ��ν���ʱ������Ӧ��CuS+H2O2+H2SO4![]() CuSO4+S+2H2O���������ж����壬��ѡ��Aװ�á�
CuSO4+S+2H2O���������ж����壬��ѡ��Aװ�á�
��2���ڶ��ν���ʱ����ʢ����Һ1�ķ�Ӧ���м���ϡ���ᣬ��������������Һ����˳���෴�������H2O2���������Ӵ��ֽ⡣����2����Ҫ�ɷ��Ƕ������衣
��3��Ũ������п������ͭ��Һʹ�õ�����������������
��4��15.0 g�ϴ����к���ͭ�����ʵ���Ϊ15.0g��12.8%��96g/mol=0.02mol��1.5gCuSO4�q5H2O��ͭ�����ʵ���Ϊ1.5g��250g/mol=0.006mol����ͭ�Ļ�����Ϊ0.006mol/0.02mol��100%=30%��