��Ŀ����
ʵ���������Ҵ���Ũ���ᷴӦ������ϩ��������������֮��Ӧ����1��2-�������飮
���Ʊ����������ڲ����Ҵ���Ũ���������������CO2��SO2����������Br2��Ӧ����HBr���������壮
��֪��CH3CH2OH
CH2=CH2��+H2O
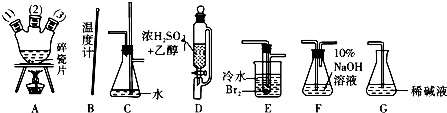
��1����������������������������Ϊԭ���Ʊ�1��2-�������飮�����������Ϊ�����ң���ȷ������˳���ǣ��̽ӿڻ���Ƥ�ܾ�����ȥ���� ��A��1������A�У� ��A��2����A��3���� �� �� �� ��
��2��װ��C�������� ��װ��G������ ��
��3��װ��F��ʢ��10%NaOH��Һ�������� ��
��4���ڷ�Ӧ��E�н��е���Ҫ��Ӧ�Ļ�ѧ����ʽΪ ��
��5����Ӧ��E�м�������ˮ���ѷ�Ӧ��E����ʢ����ˮ��С�ձ���Ŀ���ǣ� ��
��6����������ʵ���������ƿ�з�Һ����ȷ������ ��
A����Һ����ȴ������ˮ����
B����Һ����ȴ����շ�Һ����
C����ˮ������ƿ��ϡ�ͺ���շ�Һ���У�
���Ʊ����������ڲ����Ҵ���Ũ���������������CO2��SO2����������Br2��Ӧ����HBr���������壮
��֪��CH3CH2OH
| ŨH2SO4 |
| 170�� |

��1����������������������������Ϊԭ���Ʊ�1��2-�������飮�����������Ϊ�����ң���ȷ������˳���ǣ��̽ӿڻ���Ƥ�ܾ�����ȥ����
��2��װ��C��������
��3��װ��F��ʢ��10%NaOH��Һ��������
��4���ڷ�Ӧ��E�н��е���Ҫ��Ӧ�Ļ�ѧ����ʽΪ
��5����Ӧ��E�м�������ˮ���ѷ�Ӧ��E����ʢ����ˮ��С�ձ���Ŀ���ǣ�
��6����������ʵ���������ƿ�з�Һ����ȷ������
A����Һ����ȴ������ˮ����
B����Һ����ȴ����շ�Һ����
C����ˮ������ƿ��ϡ�ͺ���շ�Һ���У�
���㣺�Ҵ�����ȥ��Ӧ
ר�⣺ʵ�������
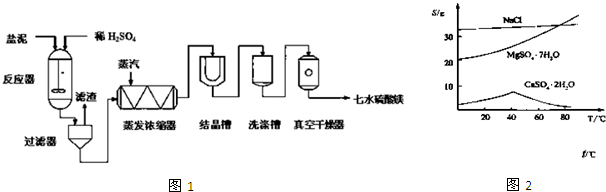
�������ڷ�����������Ҫץס���㣺��һ�DZ����ȥ������ϩ�е��������ʣ�������SO2���壬�Է�ֹSO2��Br2������ӦSO2+Br2+2H2O=2HBr+H2SO4��Ӱ��1��2-���������Ʒ���Ʊ�������DZ���������Ļ����������װ����IJ�̫��Ϥ��ʵ����������������ƿA����ѹ��Һ©��D����ȫƿ������װ��C������Ӧ��E����ˮ�������Ǿ���������Ļӷ���������װ˳���ǣ���ȡ��ϩ���壨��A��B��D��װ��һ��ȫƿ��C�����������壨F����NaOH��Һ����CO2��SO2�������������壩���Ʊ�1��2-�����������Ҫ��Ӧװ�ã�E����β��������G����������л���Ľṹ�������Լ���ĿҪ��ɽ����⣮
���
�⣺��1��������װ˳���ǣ���ȡ��ϩ���壨��A��B��D��װ��B��A��1������A�У�D��A��2����һA��3������ȫƿ��C��������������������壨F����NaOH��Һ����CO2��SO2�������������壩���Ʊ�1��2-�����������Ҫ��Ӧװ�ã�E����β��������G������
�ʴ�Ϊ��B��D��C��F��E��G��
��2��װ��C��������������ȫƿ�����ӷ����ж���Ϊ��ֹ��Ⱦ������Ӧ����β�����������ü�Һ���գ�
�ʴ�Ϊ����ȫƿ�����ջӷ�����������������ֹ��Ⱦ������
��3���Ҵ���Ũ�������ÿ�����CO2��SO2��Ϊ��ֹSO2��Br2������ӦSO2+Br2+2H2O=2HBr+H2SO4��Ӱ��1��2-���������Ʒ���Ʊ���Ӧ�ó�ȥ����ƿ�в�����CO2��SO2��
�ʴ�Ϊ����ȥ��ϩ�д�������������CO2��SO2��
��4����Ӧ��E�н��е���Ҫ��Ӧ����ϩ����ˮ�����ӳɷ�Ӧ������ʽΪ��CH2=CH2+Br2��CH2Br-CH2Br��
�ʴ�Ϊ��CH2=CH2+Br2��CH2Br-CH2Br��
��5�����ӷ�����ʢ����ˮ��С�ձ��У����Լ�����Ļӷ���ʧ��
�ʴ�Ϊ�����ӷ������������Լ�����Ļӷ���ʧ��
��6����Һ�¶Ƚϸߣ�Ӧ����ȴ���ٴ�������ƿ�к���Ũ���ᣬ���и�ʴ�ԣ�����Ҫ���д�������ѡB��
�ʴ�Ϊ��B��D��C��F��E��G��
��2��װ��C��������������ȫƿ�����ӷ����ж���Ϊ��ֹ��Ⱦ������Ӧ����β�����������ü�Һ���գ�
�ʴ�Ϊ����ȫƿ�����ջӷ�����������������ֹ��Ⱦ������
��3���Ҵ���Ũ�������ÿ�����CO2��SO2��Ϊ��ֹSO2��Br2������ӦSO2+Br2+2H2O=2HBr+H2SO4��Ӱ��1��2-���������Ʒ���Ʊ���Ӧ�ó�ȥ����ƿ�в�����CO2��SO2��
�ʴ�Ϊ����ȥ��ϩ�д�������������CO2��SO2��
��4����Ӧ��E�н��е���Ҫ��Ӧ����ϩ����ˮ�����ӳɷ�Ӧ������ʽΪ��CH2=CH2+Br2��CH2Br-CH2Br��
�ʴ�Ϊ��CH2=CH2+Br2��CH2Br-CH2Br��
��5�����ӷ�����ʢ����ˮ��С�ձ��У����Լ�����Ļӷ���ʧ��
�ʴ�Ϊ�����ӷ������������Լ�����Ļӷ���ʧ��
��6����Һ�¶Ƚϸߣ�Ӧ����ȴ���ٴ�������ƿ�к���Ũ���ᣬ���и�ʴ�ԣ�����Ҫ���д�������ѡB��
���������⿼���л���ĺϳ�ʵ����ƣ������Ĺؼ��ǰ����Ʊ�ʵ���ԭ��������ȷ���ʵ��˳���ι̰���ʵ���������ʱ��������Ŀ�ı�֤����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��У��������ĩ��̾�ϵ�д�
��У��������ĩ��̾�ϵ�д�
�����Ŀ
ʹ��һ���Լ����ܼ��������һ�������ǣ�������
| A���ױ� ��ϩ �� �Ҵ� |
| B���ױ� ��ϩ CCl4 �Ҵ� |
| C�������� �� ������ �Ҵ� |
| D���� �ױ� ���ױ� �Ҵ� |
���й���Ԫ�����ڱ���˵���У�������ǣ�������
| A��Ԫ�����ڱ���Ԫ�ذ�ԭ��������С�������ж��ɵ� |
| B��Ԫ��ԭ�ӵĵ��Ӳ����������������ڵ��������� |
| C���ġ��塢�������ж�����18��Ԫ�� |
| D��Ԫ�����ڱ�����18�С�16���� |
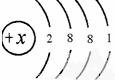 ��֪��ԭ�ӵ�ԭ�ӽṹʾ��ͼΪ������ͼ��ʾ��
��֪��ԭ�ӵ�ԭ�ӽṹʾ��ͼΪ������ͼ��ʾ��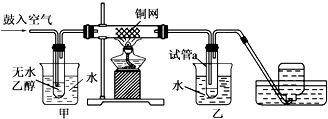
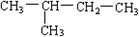 ��
��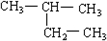
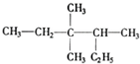
 ?2��2-�������飮
?2��2-�������飮